当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Divergent JNK Phosphorylation of HDAC3 in Triple-Negative Breast Cancer Cells Determines HDAC Inhibitor Binding and Selectivity
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1016/j.chembiol.2017.08.015 Thomas W. Hanigan , Shaimaa M. Aboukhatwa , Taha Y. Taha , Jonna Frasor , Pavel A. Petukhov
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1016/j.chembiol.2017.08.015 Thomas W. Hanigan , Shaimaa M. Aboukhatwa , Taha Y. Taha , Jonna Frasor , Pavel A. Petukhov
|
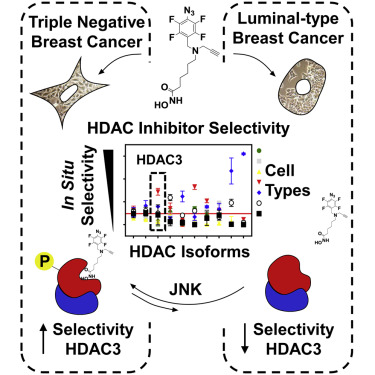
Histone deacetylase (HDAC) catalytic activity is regulated by formation of co-regulator complexes and post-translational modification. Whether these mechanisms are transformed in cancer and how this affects the binding and selectivity of HDAC inhibitors (HDACis) is unclear. In this study, we developed a method that identified a 3- to 16-fold increase in HDACi selectivity for HDAC3 in triple-negative breast cancer (TNBC) cells in comparison with luminal subtypes that was not predicted by current practice measurements with recombinant proteins. We found this increase was caused by c-Jun N-terminal kinase (JNK) phosphorylation of HDAC3, was independent of HDAC3 complex composition or subcellular localization, and was associated with a 5-fold increase in HDAC3 enzymatic activity. This study points to HDAC3 and the JNK axes as targets in TNBC, highlights how HDAC phosphorylation affects HDACi binding and selectivity, and outlines a method to identify changes in individual HDAC isoforms catalytic activity, applicable to any disease state.
中文翻译:

在三阴性乳腺癌细胞中HDAC3的不同JNK磷酸化决定了HDAC抑制剂的结合和选择性
组蛋白脱乙酰基酶(HDAC)催化活性是通过形成共调节复合物和翻译后修饰来调节的。这些机制是否会在癌症中转化,以及如何影响HDAC抑制剂(HDACis)的结合和选择性尚不清楚。在这项研究中,我们开发了一种方法,该方法与三联阴性乳腺癌(TNBC)细胞中的HDAC3对HDAC3的选择性相比,目前的重组蛋白尚无法预测的管腔亚型提高了3至16倍。我们发现这种增加是由HDAC3的c-Jun N末端激酶(JNK)磷酸化引起的,与HDAC3复杂成分或亚细胞定位无关,并且与HDAC3酶活性增加5倍有关。这项研究指出,HDAC3和JNK轴是TNBC的目标,
更新日期:2017-11-19
中文翻译:

在三阴性乳腺癌细胞中HDAC3的不同JNK磷酸化决定了HDAC抑制剂的结合和选择性
组蛋白脱乙酰基酶(HDAC)催化活性是通过形成共调节复合物和翻译后修饰来调节的。这些机制是否会在癌症中转化,以及如何影响HDAC抑制剂(HDACis)的结合和选择性尚不清楚。在这项研究中,我们开发了一种方法,该方法与三联阴性乳腺癌(TNBC)细胞中的HDAC3对HDAC3的选择性相比,目前的重组蛋白尚无法预测的管腔亚型提高了3至16倍。我们发现这种增加是由HDAC3的c-Jun N末端激酶(JNK)磷酸化引起的,与HDAC3复杂成分或亚细胞定位无关,并且与HDAC3酶活性增加5倍有关。这项研究指出,HDAC3和JNK轴是TNBC的目标,


























 京公网安备 11010802027423号
京公网安备 11010802027423号