当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bromide-Mediated C–H Bond Functionalization: Intermolecular Annulation of Phenylethanone Derivatives with Alkynes for the Synthesis of 1-Naphthols
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03186 Tao Lu 1 , Ya-Ting Jiang 1 , Feng-Ping Ma 1 , Zi-Jing Tang 1 , Liu Kuang 1 , Yu-Xuan Wang 1 , Bin Wang 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03186 Tao Lu 1 , Ya-Ting Jiang 1 , Feng-Ping Ma 1 , Zi-Jing Tang 1 , Liu Kuang 1 , Yu-Xuan Wang 1 , Bin Wang 1
Affiliation
|
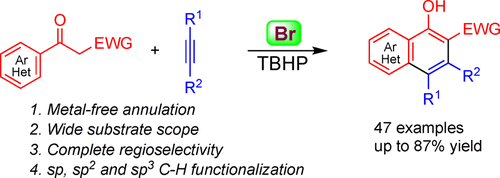
Bromide-mediated intermolecular annulation of phenylethanone derivatives with alkynes has been developed, which allows for the regioselective formation of polysubstituted 1-naphthols. The usage of readily available bromine catalyst, broad substrate scope, and mild conditions make this protocol very practical. Mechanistic investigations reveal that the bromination of phenylethanone derivatives occurs to yield bromo-substituted intermediates, which react in situ with alkynes to furnish the desired 1-naphthols.
中文翻译:

溴化物介导的CH键功能化:苯乙酮衍生物与炔烃的分子间环化反应,用于合成1-萘酚
已经开发了由溴化物介导的苯乙酮衍生物与炔烃的分子间环化反应,这种反应可以区域选择性地形成多取代的1-萘酚。易于使用的溴催化剂的使用,广泛的底物范围和温和的条件使该方案非常实用。机理研究表明,发生了苯乙酮衍生物的溴化反应,生成了溴取代的中间体,该中间体与炔烃就地反应,生成了所需的1-萘酚。
更新日期:2017-11-17
中文翻译:

溴化物介导的CH键功能化:苯乙酮衍生物与炔烃的分子间环化反应,用于合成1-萘酚
已经开发了由溴化物介导的苯乙酮衍生物与炔烃的分子间环化反应,这种反应可以区域选择性地形成多取代的1-萘酚。易于使用的溴催化剂的使用,广泛的底物范围和温和的条件使该方案非常实用。机理研究表明,发生了苯乙酮衍生物的溴化反应,生成了溴取代的中间体,该中间体与炔烃就地反应,生成了所需的1-萘酚。



























 京公网安备 11010802027423号
京公网安备 11010802027423号