当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of the Benzodioxole Group in the Interactions between the Natural Alkaloids Chelerythrine and Coptisine and the Human Telomeric G-Quadruplex DNA. A Multiapproach Investigation
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00350 F. Papi 1, 2 , M. Ferraroni 1 , R. Rigo 3 , S. Da Ros 3 , C. Bazzicalupi 1 , C. Sissi 3 , P. Gratteri 2
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00350 F. Papi 1, 2 , M. Ferraroni 1 , R. Rigo 3 , S. Da Ros 3 , C. Bazzicalupi 1 , C. Sissi 3 , P. Gratteri 2
Affiliation
|
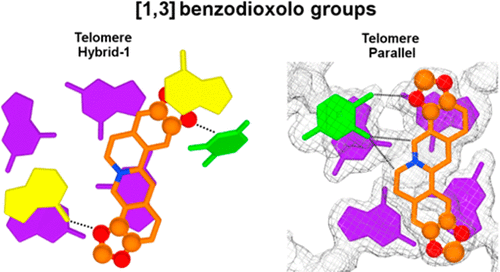
The binding properties toward the human telomeric G-quadruplex of the two natural alkaloids coptisine and chelerythrine were studied using spectroscopic techniques, molecular modeling, and X-ray diffraction analysis. The results were compared with reported data for the parent compounds berberine and sanguinarine. Spectroscopic studies showed modest, but different rearrangements of the DNA–ligand complexes, which can be explained considering particular stereochemical features for these alkaloids, in spite of the similarity of their skeletons. In fact, the presence of a dioxolo moiety rather than the two methoxy functions improves the efficiency of coptisine and sanguinarine in comparison to berberine and chelerythrine, and the overall stability trend is sanguinarine > chelerythrine ≈ coptisine > berberine. Accordingly, the X-ray diffraction analysis confirmed the involvement of the benzodioxolo groups in the coptisine/DNA binding by means of π···π, O···π, and CH···O interactions. Similar information is provided by modeling studies, which, additionally, evidenced reasons for the quadruplex vs double-helix selectivity shown by these alkaloids. Thus, the analyses shed light on the key role of the benzodioxolo moieties in strengthening the interaction with the G4-folded human telomeric sequence and indicated the superior G4 stabilizing properties of the benzophenanthridine scaffold with respect to the protoberberine one and conversely the better G4 vs dsDNA selectivity profile of coptisine over the other alkaloids.
中文翻译:

苯并二恶唑基在天然生物碱白屈菜红碱和黄连碱与人端粒G-四链体DNA之间的相互作用中的作用。多途径调查
两种天然生物碱黄连碱和白屈菜红碱对人端粒G-四联体的结合特性使用光谱技术,分子建模和X射线衍射分析进行了研究。将结果与母体化合物小ber碱和血红素的报道数据进行了比较。光谱研究显示,DNA-配体复合物适度但不同的重排,尽管它们的骨架相似,但考虑到这些生物碱的特殊立体化学特征也可以解释。实际上,与小ber碱和白屈菜红碱相比,存在二氧杂酚基团而不是两个甲氧基官能团可以提高黄连碱和血红素碱的效率,并且总体稳定性趋势是血红素碱>白屈菜红碱≈黄连碱>小ber碱。因此,X射线衍射分析证实了苯并二恶唑基团通过π···π,O···π和CH··O的相互作用参与了黄连碱/ DNA的结合。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。
更新日期:2017-11-17
中文翻译:

苯并二恶唑基在天然生物碱白屈菜红碱和黄连碱与人端粒G-四链体DNA之间的相互作用中的作用。多途径调查
两种天然生物碱黄连碱和白屈菜红碱对人端粒G-四联体的结合特性使用光谱技术,分子建模和X射线衍射分析进行了研究。将结果与母体化合物小ber碱和血红素的报道数据进行了比较。光谱研究显示,DNA-配体复合物适度但不同的重排,尽管它们的骨架相似,但考虑到这些生物碱的特殊立体化学特征也可以解释。实际上,与小ber碱和白屈菜红碱相比,存在二氧杂酚基团而不是两个甲氧基官能团可以提高黄连碱和血红素碱的效率,并且总体稳定性趋势是血红素碱>白屈菜红碱≈黄连碱>小ber碱。因此,X射线衍射分析证实了苯并二恶唑基团通过π···π,O···π和CH··O的相互作用参与了黄连碱/ DNA的结合。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。建模研究提供了类似的信息,此外,这些生物碱显示出四链体与双螺旋选择性的有据可查的原因。因此,分析揭示了苯并二恶唑部分在增强与G4折叠的人类端粒序列的相互作用中的关键作用,并表明了苯并菲啶骨架相对于原小ber碱具有更好的G4稳定特性,反之,更好的是G4与dsDNA黄连对其他生物碱的选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号