当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Role of Structural Order in the Transformation of Electrical Conductivity in Ca2FeCoO6−δ, CaSrFeCoO6−δ, and Sr2FeCoO6−δ
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02079 Ram Krishna Hona 1 , Ashfia Huq 2 , Farshid Ramezanipour 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02079 Ram Krishna Hona 1 , Ashfia Huq 2 , Farshid Ramezanipour 1
Affiliation
|
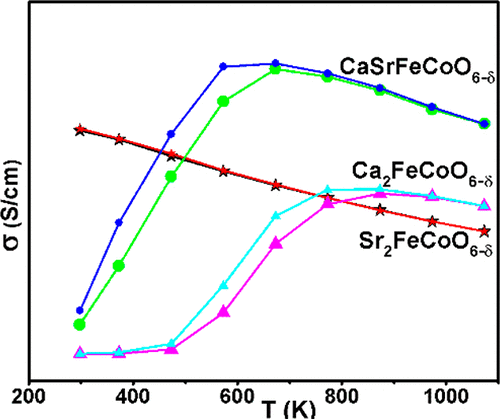
The ability to control the electrical conductivity of solid-state oxides using structural parameters has been demonstrated. A correlation has been established between the electrical conductivity and structural order in a series of oxygen-deficient perovskites using X-ray and neutron diffraction, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and electrical conductivity studies at a wide temperature range, 25–800 °C. The crystal structure of CaSrFeCoO6−δ has been determined, and its stark contrast to Ca2FeCoO6−δ and Sr2FeCoO6−δ has been demonstrated. The Fe/Co distribution over tetrahedral and octahedral sites has been determined using neutron diffraction. There is a systematic increase in the structural order in progression from Sr2FeCoO6−δ (δ = 0.5) to CaSrFeCoO6−δ (δ = 0.8) and Ca2FeCoO6−δ (δ = 0.9) . The oxygen contents of these materials were determined using iodometric titration and TGA. At room temperature, there is an inverse correlation between the electrical conductivity and structural order. The ordered Ca2 and CaSr compounds are semiconductors, while the disordered Sr2 compund shows metallic behavior. The metallic nature of the Sr2 material persists up to 1073 K (800 °C), while the Ca2 and CaSr compounds undergo a semiconductor-to-metal transition above 500 and 300 °C, respectively, highlighting another important impact of the structural order. At high temperature, the CaSr compound has the highest conductivity compared to the Ca2 and Sr2 materials. There appears to be an optimum degree of structural order that leads to the highest conductivity at high temperature. Another consequence of the structural order is the observation of mixed ionic–electronic conductivity in CaSr and Ca2 compounds, as is evident from the hysteresis in the conductivity data obtained during heating and cooling cycles. The average ionic radius required for each structural transition was determined through the synthesis of 21 different materials by systematic variation of the Ca/Sr ratio. In addition, SEM and XPS were employed to gain insight into the crystallite morphology and oxidation states of transition metals, revealing an interesting redox process between Fe and Co.
中文翻译:

揭示结构顺序在Ca 2 FeCoO 6−δ,CaSrFeCoO 6−δ和Sr 2 FeCoO 6−δ的电导率转变中的作用
已经证明了使用结构参数控制固态氧化物电导率的能力。使用X射线和中子衍射,X射线光电子能谱(XPS),扫描电子显微镜(SEM),热重分析(TGA),和电导率研究在25–800°C的较宽温度范围内进行。确定了CaSrFeCoO6 -δ的晶体结构,并与Ca 2 FeCoO6 -δ和Sr 2 FeCoO6 -δ形成了鲜明的对比已经证明。已使用中子衍射法确定了四面体和八面体位点上的Fe / Co分布。从Sr 2 FeCoO 6−δ(δ= 0.5)到CaSrFeCoO 6−δ(δ= 0.8)和Ca 2 FeCoO 6−δ(δ= 0.9)的结构顺序有系统地增加。使用碘量滴定法和TGA测定这些材料的氧含量。在室温下,电导率与结构顺序之间呈反比关系。有序的Ca 2和CaSr化合物是半导体,而无序的Sr 2化合物则表现出金属行为。Sr 2的金属性质这种材料可在高达1073 K(800°C)的温度下持续存在,而Ca 2和CaSr化合物则分别在500和300°C以上经历半导体到金属的转变,这突显了结构顺序的另一个重要影响。与Ca 2和Sr 2材料相比,在高温下,CaSr化合物具有最高的电导率。似乎存在最佳程度的结构顺序,可以在高温下实现最高的电导率。结构顺序的另一个结果是在CaSr和Ca 2中观察到混合的离子-电子电导率从加热和冷却循环中获得的电导率数据中的磁滞可以明显看出这种化合物。每个结构转变所需的平均离子半径是通过Ca / Sr比的系统变化,通过合成21种不同材料来确定的。此外,使用SEM和XPS深入了解了过渡金属的微晶形态和氧化态,揭示了Fe和Co之间有趣的氧化还原过程。
更新日期:2017-11-17
中文翻译:

揭示结构顺序在Ca 2 FeCoO 6−δ,CaSrFeCoO 6−δ和Sr 2 FeCoO 6−δ的电导率转变中的作用
已经证明了使用结构参数控制固态氧化物电导率的能力。使用X射线和中子衍射,X射线光电子能谱(XPS),扫描电子显微镜(SEM),热重分析(TGA),和电导率研究在25–800°C的较宽温度范围内进行。确定了CaSrFeCoO6 -δ的晶体结构,并与Ca 2 FeCoO6 -δ和Sr 2 FeCoO6 -δ形成了鲜明的对比已经证明。已使用中子衍射法确定了四面体和八面体位点上的Fe / Co分布。从Sr 2 FeCoO 6−δ(δ= 0.5)到CaSrFeCoO 6−δ(δ= 0.8)和Ca 2 FeCoO 6−δ(δ= 0.9)的结构顺序有系统地增加。使用碘量滴定法和TGA测定这些材料的氧含量。在室温下,电导率与结构顺序之间呈反比关系。有序的Ca 2和CaSr化合物是半导体,而无序的Sr 2化合物则表现出金属行为。Sr 2的金属性质这种材料可在高达1073 K(800°C)的温度下持续存在,而Ca 2和CaSr化合物则分别在500和300°C以上经历半导体到金属的转变,这突显了结构顺序的另一个重要影响。与Ca 2和Sr 2材料相比,在高温下,CaSr化合物具有最高的电导率。似乎存在最佳程度的结构顺序,可以在高温下实现最高的电导率。结构顺序的另一个结果是在CaSr和Ca 2中观察到混合的离子-电子电导率从加热和冷却循环中获得的电导率数据中的磁滞可以明显看出这种化合物。每个结构转变所需的平均离子半径是通过Ca / Sr比的系统变化,通过合成21种不同材料来确定的。此外,使用SEM和XPS深入了解了过渡金属的微晶形态和氧化态,揭示了Fe和Co之间有趣的氧化还原过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号