当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction monitoring reveals poisoning mechanism of Pd2(dba)3 and guides catalyst selection
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1039/c7cc08018b Chiara Colletto 1, 2, 3, 4 , Jordi Burés 1, 2, 3, 4 , Igor Larrosa 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1039/c7cc08018b Chiara Colletto 1, 2, 3, 4 , Jordi Burés 1, 2, 3, 4 , Igor Larrosa 1, 2, 3, 4
Affiliation
|
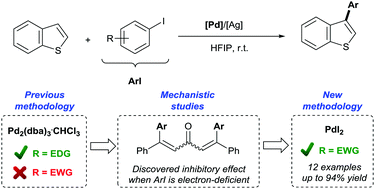
We have discovered that the dba ligand in the commonly used Pd2(dba)3·CHCl3 cross-coupling pre-catalyst is susceptible to bis-arylation when used in the presence of aryl iodides. The in situ formed dbaAr2 ligands result in Pd-species with altered catalytic activity. In the case of study, the room temperature C3 arylation of benzo[b]thiophenes with aryl iodides, we have observed a marked catalyst deactivation when dba is arylated with electron-deficient aryl iodides, accounting for the poor yields obtained in the C3 arylation reactions with these aryl iodides. Based on these studies, we report a new catalytic system, employing a dba-free Pd catalyst, which allows for the first time the direct C3 arylation of benzo[b]thiophenes with electron-deficient aryl iodides at room temperature.
中文翻译:

反应监测揭示了Pd 2(dba)3的中毒机理并指导催化剂的选择
我们已经发现,当在芳基碘化物存在下使用时,常用的Pd 2(dba)3 ·CHCl 3交联预催化剂中的dba配体易于双芳基化。在原位形成dbaAr 2配体导致的Pd-物种与改变的催化活性。在研究的情况下,室温下苯并[ b]的C3芳基化]噻吩与芳基碘化物的反应,我们发现当dba与缺电子的芳基碘化物进行芳基化时,催化剂会明显失活,这说明在这些芳基碘化物的C3芳基化反应中收率低。基于这些研究,我们报告了一种新的催化体系,该体系采用不含dba的Pd催化剂,这首次允许在室温下苯并[ b ]噻吩与缺电子的芳基碘化物直接进行C3芳基化。
更新日期:2017-12-01
中文翻译:

反应监测揭示了Pd 2(dba)3的中毒机理并指导催化剂的选择
我们已经发现,当在芳基碘化物存在下使用时,常用的Pd 2(dba)3 ·CHCl 3交联预催化剂中的dba配体易于双芳基化。在原位形成dbaAr 2配体导致的Pd-物种与改变的催化活性。在研究的情况下,室温下苯并[ b]的C3芳基化]噻吩与芳基碘化物的反应,我们发现当dba与缺电子的芳基碘化物进行芳基化时,催化剂会明显失活,这说明在这些芳基碘化物的C3芳基化反应中收率低。基于这些研究,我们报告了一种新的催化体系,该体系采用不含dba的Pd催化剂,这首次允许在室温下苯并[ b ]噻吩与缺电子的芳基碘化物直接进行C3芳基化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号