当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extraordinary Long-Term-Stability in Kinetically Stabilized Amorphous Solid Dispersions of Fenofibrate
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00735 Frank Theil 1 , Johanna Milsmann 1 , Samuel O. Kyeremateng 1 , Sankaran Anantharaman 1 , Jörg Rosenberg 1 , Holger van Lishaut 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00735 Frank Theil 1 , Johanna Milsmann 1 , Samuel O. Kyeremateng 1 , Sankaran Anantharaman 1 , Jörg Rosenberg 1 , Holger van Lishaut 1
Affiliation
|
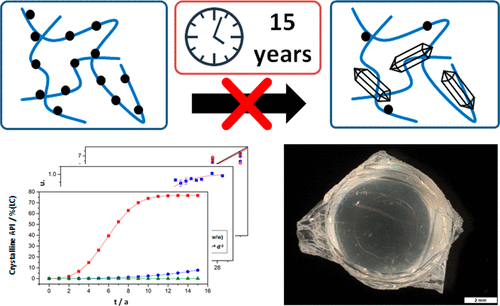
Inhibition of recrystallization of the drug substance in kinetically stabilized amorphous solid dispersions (ASDs) within and beyond shelf life is still a matter of debate. Generally, these ASD systems are considered to be prone to recrystallization, but examples of their long-term stability are emerging in the literature. Since, in some cases, the formation of crystals may impact bioavailability, recrystallization may present a relevant risk for patients as it potentially lowers the effective dose of the formulation. This study shows that such metastable formulations may indeed remain amorphous even after 15 years of storage under ambient conditions. A formulation of fenofibrate stored for 15 years was compared to a freshly prepared batch. A complete physicochemical characterization regarding content, purity, water content and glass transition was conducted. The emphasis of this physicochemical characterization was on crystallinity as a critical quality attribute: polarized light microscopy (PLM) was used as the standard qualitative method and X-ray powder diffraction (XRPD) as the standard quantitative method. An investigation of the crystal growth kinetics by transmission Raman spectroscopy (TRS) was conducted to build a predictive model. The model was applied successfully to predict the observed physical state of the 15-year-old samples. The observations presented here demonstrate that kinetic stabilization alone is able to prevent crystallization in ASDs over prolonged storage periods, suggesting the need for a reassessment of the risk perception for this kind of ASD formulations.
中文翻译:

非诺贝特的动稳定非晶固体分散体的非凡长期稳定性
货架期内外的动力学稳定的无定形固体分散体(ASD)中药物重结晶的抑制作用仍是争论的焦点。通常,这些ASD系统被认为容易重结晶,但是其长期稳定性的例子却在文献中不断涌现。由于在某些情况下,晶体的形成可能会影响生物利用度,因此重结晶可能会给患者带来相关风险,因为重结晶可能会降低制剂的有效剂量。这项研究表明,即使在环境条件下保存15年后,此类亚稳制剂也可能确实保持无定形。将保存了15年的非诺贝特的制剂与新制备的批次进行了比较。关于含量,纯度,进行水含量和玻璃化转变。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化能够防止ASD在延长的储存期内结晶,这表明需要重新评估此类ASD制剂的风险感知。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化能够防止ASD在延长的储存期内结晶,这表明需要重新评估此类ASD制剂的风险感知。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。
更新日期:2017-11-16
中文翻译:

非诺贝特的动稳定非晶固体分散体的非凡长期稳定性
货架期内外的动力学稳定的无定形固体分散体(ASD)中药物重结晶的抑制作用仍是争论的焦点。通常,这些ASD系统被认为容易重结晶,但是其长期稳定性的例子却在文献中不断涌现。由于在某些情况下,晶体的形成可能会影响生物利用度,因此重结晶可能会给患者带来相关风险,因为重结晶可能会降低制剂的有效剂量。这项研究表明,即使在环境条件下保存15年后,此类亚稳制剂也可能确实保持无定形。将保存了15年的非诺贝特的制剂与新制备的批次进行了比较。关于含量,纯度,进行水含量和玻璃化转变。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化能够防止ASD在延长的储存期内结晶,这表明需要重新评估此类ASD制剂的风险感知。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化能够防止ASD在延长的储存期内结晶,这表明需要重新评估此类ASD制剂的风险感知。此物理化学表征的重点是结晶度,这是关键的质量属性:偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。偏光显微镜(PLM)被用作标准定性方法,X射线粉末衍射(XRPD)被用作标准定量方法。通过透射拉曼光谱法(TRS)对晶体生长动力学进行了研究,以建立预测模型。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。该模型已成功应用于预测15岁样本的观察到的身体状态。此处提出的观察结果表明,单独的动力学稳定化作用能够防止ASD在延长的储存期内结晶,这表明需要对这种ASD制剂的风险感知进行重新评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号