当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study
The Lancet Neurology ( IF 48.0 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1474-4422(17)30369-1 James F Howard , Kimiaki Utsugisawa , Michael Benatar , Hiroyuki Murai , Richard J Barohn , Isabel Illa , Saiju Jacob , John Vissing , Ted M Burns , John T Kissel , Srikanth Muppidi , Richard J Nowak , Fanny O'Brien , Jing-Jing Wang , Renato Mantegazza , Claudio Gabriel Mazia , Miguel Wilken , Carolina Ortea , Juliet Saba , Marcelo Rugiero , Mariela Bettini , Gonzalo Vidal , Alejandra Dalila Garcia , Phillipa Lamont , Wai-Kuen Leong , Heidi Boterhoven , Beverly Fyfe , Leslie Roberts , Mahi Jasinarachchi , Natasha Willlems , Julia Wanschitz , Wolfgang Löscher , Jan De Bleecker , Guy Van den Abeele , Kathy de Koning , Katrien De Mey , Rudy Mercelis , Linda Wagemaekers , Delphine Mahieu , Philip Van Damme , Charlotte Smetcoren , Olivier Stevens , Sarah Verjans , Ann D'Hondt , Petra Tilkin , Alzira Alves de Siqueira Carvalho , Rosa Hasan , Igor Dias Brockhausen , David Feder , Daniel Ambrosio , Ana Paula Melo , Rosana Rocha , Bruno Rosa , Thabata Veiga , Luiz Augusto da Silva , Jordana Gonçalves Geraldo , Maria da Penha Morita Ananias , Erica Nogueira Coelho , Gabriel Paiva , Marina Pozo , Natalia Prando , Debora Dada Martineli Torres , Cristiani Fernanda Butinhao , Erica Coelho , Luciana Renata Cubas Volpe , Gustavo Duran , Tamires Cristina Gomes da Silva , Luiz Otavio Maia Gonçalves , Lucas Eduardo Pazetto , Luciana Souza Duca , Tomás Augusto Suriane Fialho , Maurício André Gheller Friedrich , Alexandre Guerreiro , Henrique Mohr , Maurer Pereira Martins , Daiane da Cruz Pacheco , Ana Paula Macagnan , Aline de Cassia Santos , Acary Souza Bulle Oliveira , Ana Carolina Amaral de Andrade , Marcelo Annes , Valeria Cavalcante Lino , Wladimir Pinto , Carolina Miranda , Fernanda Carrara , Iandra Souza , Angela Genge , Rami Massie , Natasha Campbell , Vera Bril , Hans Katzberg , Mehran Soltani , Eduardo Ng , Zaeem Siddiqi , Celile Phan , Derrick Blackmore , Stanislav Vohanka , Josef Bednarik , Magda Chmelikova , Marek Cierny , Stanislava Toncrova , Jana Junkerova , Barbora Kurkova , Katarina Reguliova , Olga Zapletalova , Jiri Pitha , Iveta Novakova , Michaela Tyblova , Marcela Wolfova , Ivana Jurajdova , Henning Andersen , Thomas Harbo , Lotte Vinge , Anita Mogensen , Joan Højgaard , Nanna Witting , Anne Mette Autzen , Jane Pedersen , Markus Färkkilä , Sari Atula , Anne Nyrhinen , Juha-Pekka Erälinna , Mikko Laaksonen , Olli Oksaranta , Jaana Eriksson , Tuula Harrison , Claude Desnuelle , Sabrina Sacconi , Marie-Hélène Soriani , Sonia Decressac , Julie Moutarde , Pauline Lahaut , Guilhem Solé , Gwendal Le Masson , Anne-Cécile Wielanek-Bachelet , Morgane Gaboreau , Caroline Moreau , Amy Wilson , Christophe Vial , Françoise Bouhour , Helene Gervais-Bernard , Hélène Merle , Caroline Hourquin , Arnaud Lacour , Olivier Outteryck , Patrick Vermersch , Hélène Zephir , Edouard Millois , Michel Deneve , Fabienne Deruelle , Benedikt Schoser , Stephan Wenninger , Martin Stangel , Sascha Alvermann , Stefan Gingele , Thomas Skripuletz , Kurt-Wolfram Suehs , Corinna Trebst , Karin Fricke , Sotirios Papagiannopoulos , Sevasti Bostantzopoulou , Nicholas Vlaikidis , Martha Zampaki , Nikoletta Papadopoulou , Dimos-Dimitrios Mitsikostas , Eleni Kasioti , Efstathia Mitropoulou , Despoina Charalambous , Csilla Rozsa , Melinda Horvath , Gabor Lovas , Judit Matolcsi , Gyorgyi Szabo , Brigitta Szabadosne , Laszlo Vecsei , Livia Dezsi , Edina Varga , Monika Konyane , Bella Gross , Olga Azrilin , Nelly Greenbereg , Hila Bali Kuperman , Giovanni Antonini , Matteo Garibaldi , Stefania Morino , Fernanda Troili , Antonella Di Pasquale , Alessandro Filla , Teresa Costabile , Enrico Marano , Francesco Sacca , Angela Marsil , Giorgia Puorro , Michelangelo Maestri Tassoni , Anna De Rosa , Silvia Bonanno , Carlo Antozzi , Lorenzo Maggi , Angela Campanella , Corrado Angelini , Paola Cudia , Valentina Pegoraro , Elena Pinzan , Francesca Bevilacqua , Daniele Orrico , Domenico Marco Bonifati , Amelia Evoli , Paolo Emilio Alboini , Valentina D'Amato , Raffaele Iorio , Maurizio Inghilleri , Laura Fionda , Vittorio Frasca , Elena Giacomelli , Maria Gori , Diego Lopergolo , Emanuela Onesti , Maria Gabriele , Francesco Patti , Andrea Salvatore Caramma , Silvia Messina , Ester Reggio , Cinzia Caserta , Akiyuki Uzawa , Tetsuya Kanai , Masahiro Mori , Yoko Kaneko , Akiko Kanzaki , Eri Kobayashi , Katsuhisa Masaki , Dai Matsuse , Takuya Matsushita , Taira Uehara , Misa Shimpo , Maki Jingu , Keiko Kikutake , Yumiko Nakamura , Yoshiko Sano , Yuriko Nagane , Ikuko Kamegamori , Yuko Fujii , Kazumi Futono , Tomoko Tsuda , Yuka Saito , Hidekazu Suzuki , Miyuki Morikawa , Makoto Samukawa , Sachiko Kamakura , Hirokazu Shiraishi , Teiichiro Mitazaki , Masakatsu Motomura , Akihiro Mukaino , Shunsuke Yoshimura , Shizuka Asada , Tomomi Kobashikawa , Megumi Koga , Yasuko Maeda , Kazumi Takada , Mihoko Takada Takada , Yumi Yamashita , Seiko Yoshida , Yasushi Suzuki , Tetsuya Akiyama , Koichi Narikawa , Kenichi Tsukita , Fumie Meguro , Yusuke Fukuda , Miwako Sato , Hidenori Matsuo , Takayasu Fukudome , Yuichiro Gondo , Yasuhiro Maeda , Akiko Nagaishi , Shunya Nakane , Yoshinori Okubo , Meinoshin Okumura , Soichiro Funaka , Tomohiro Kawamura , Masayuki Makamori , Masanori Takahashi , Tomoya Hasuike , Eriko Higuchi , Hisako Kobayashi , Kaori Osakada , Namie Taichi , Emiko Tsuda , Takashi Hayashi , Shin Hisahara , Tomihiro Imai , Jun Kawamata , Takashi Murahara , Masaki Saitoh , Shun Shimohama , Shuichiro Suzuki , Daisuke Yamamoto , Shingo Konno , Tomomi Imamura , Masashi Inoue , Mayumi Murata , Hiroshi Nakazora , Ritsu Nakayama , Yasuko Ikeda , Miki Ogawa , Maoko Shirane , Takashi Kanda , Motoharu Kawai , Michiaki Koga , Junichi Ogasawara , Masatoshi Omoto , Yasuteru Sano , Hideki Arima , Sachie Fukui , Shigemi Shimose , Hirokazu Shinozaki , Masanori Watanabe , Chieko Yoshikawa , Anneke van der Kooi , Marianne de Visser , Tamar Gibson , Jos Maessen , Marc de Baets , Catherine Faber , Maria Johanna Keijzers , Monique Miesen , Anna Kostera-Pruszczyk , Anna Kaminska , Byung-Jo Kim , Chang Nyoung Lee , Yong Seo Koo , Hung Youl Seok , Hoo Nam Kang , HyeJin Ra , Byoung Joon Kim , Eun Bin Cho , HyeLim Lee , Ju-Hong Min , Jinmyoung Seok , Da Yoon Koh , JuYoung Kwon , JiEun Lee , SangAe Park , Yoon-Ho Hong , Jae-Sung Lim , MiRi Kim , Seung Min Kim , Yool-hee Kim , Hyung Seok Lee , Ha Young Shin , Eun Bi Hwang , MiJu Shin , Denis Sazonov , Asya Yarmoschuk , Larisa Babenko , Nadezhda Malkova , Anna Melnikova , Denis Korobko , Evgeniya Kosykh , Dmitry Pokhabov , Yulia Nesterova , Vladislav Abramov , Victor Balyazin , Carlos Casasnovas Pons , Maria Alberti Aguilo , Christian Homedes-Pedret , Natalia Julia Palacios , Ana Lazaro , Exuperio Diez Tejedor , Mireya Fernandez-Fournier , Pedro Lopez Ruiz , Francisco Javier Rodriguez de Rivera , Maria Salvado Figueras , Josep Gamez , Maria Salvado , Elena Cortes Vicente , Jordi Diaz-Manera , Luis Querol Gutierrez , Ricardo Rojas Garcia , Nuria Vidal , Elisabet Arribas-Ibar , Fredrik Piehl , Albert Hietala , Lena Bjarbo , Christopher Lindberg , Daniel Jons , Blanka Andersson , Ihsan Sengun , Pinar Ozcelik , Celal Tuga , Muzeyyen Ugur , Cavit Boz , Didem Altiparmak , Sibel Gazioglu , Cigdem Ozen Aydin , Sevim Erdem-Ozdamar , Can Ebru Bekircan-Kurt , Ezgi Yilmaz , Nazire Pinar Acar , Yagmur Caliskan , Husnu Efendi , Seda Aydinlik , Hakan Cavus , Cansu Semiz , Ozlem Tun , Murat Terzi , Baki Dogan , Musa Kazim Onar , Sedat Sen , Tugce Kirbas Cavdar , Fiona Norwood , Aikaterini Dimitriou , Jakit Gollogly , Mohamed Mahdi-Rogers , Arshira Seddigh , Gal Maier , Faisal Sohail , Sivakumar Sathasivam , Heike Arndt , Debbie Davies , Dave Watling , Michael Rivner , J. Edward Hartmann , Brandy Quarles , Nicole Smalley , Anthony Amato , Thomas Cochrane , Mohammed Salajegheh , Kristen Roe , Katherine Amato , Shirli Toska , Gil Wolfe , Nicholas Silvestri , Kara Patrick , Karen Zakalik , Jonathan Katz , Robert Miller , Marguerite Engel , Elena Bravver , Benjamin Brooks , Sarah Plevka , Maryanne Burdette , Mohammad Sanjak , Megan Kramer , Joanne Nemeth , Clara Schommer , Vern Juel , Jeffrey Guptill , Lisa Hobson-Webb , Kate Beck , Donna Carnes , John Loor , Amanda Anderson , Dale Lange , Eliz Agopian , Jonathan Goldstein , Erin Manning , Lindsay Kaplan , Shara Holzberg , Nicole Kassebaum , Robert Pascuzzi , Cynthia Bodkin , John Kincaid , Riley Snook , Sandra Guinrich , Angela Micheels , Vinay Chaudhry , Andrea Corse , Betsy Mosmiller , Doreen Ho , Jayashri Srinivasan , Michael Vytopil , Nicholas Ventura , Stephanie Scala , Cynthia Carter , Craig Donahue , Carol Herbert , Elaine Weiner , Jonathan McKinnon , Laura Haar , Naya McKinnon , Karan Alcon , Kevin Daniels , Nadia Sattar , Dennis Jeffery , Kaitlyn McKenna , Amanda Guidon , William David , Christina Dheel , Mark Levine-Weinberg , Catherine Nigro , Ericka Simpson , Stanley H Appel , Eugene Lai , Luis Lay, Jr , Milvia Pleitez , Sharon Halton , Casey Faigle , Lisa Thompson , Mark Sivak , Susan Shin , Joan Bratton , Daniel Jacobs , Gavin Brown , Ibrez Bandukwala , Morris Brown , Jennifer Kane , Ira Blount , Miriam Freimer , J. Chad Hoyle , Julie Agriesti , Julie Khoury , Tessa Marburger , Harpreet Kaur , Diana Dimitrova , Michelle Mellion , George Sachs , Brigid Crabtree , Roseann Keo , Ele Kim Perez , Sandra Taber , James Gilchrist , Angela Andoin , Taylor Darnell , Neelam Goyal , Sarada Sakamuri , Yuen T So , Lesly Welsh Welsh , Ratna Bhavaraju-Sanka , Alejandro Tobon Gonzalez , Floyd Jones , Amy Saklad , Sharon Nations , Jaya Trivedi , Steve Hopkins , Mohamed Kazamel , Mohammad Alsharabati , Liang Lu , Sandi Mumfrey-Thomas , Amy Woodall , David Richman , Janelle Butters , Molly Lindsay , Tahseen Mozaffar , Tiyonnoh Cash , Namita Goyal , Gulmohor Roy , Veena Mathew , Fatima Maqsood , Brian Minton , H. James Jones , Jeffrey Rosenfeld , Rebekah Garcia , Sonia Garcia , Laura Echevarria , Michael Pulley , Shachie Aranke , Alan Ross Berger , Jaimin Shah , Yasmeen Shabbir , Lisa Smith , Mary Varghese , Laurie Gutmann , Ludwig Gutmann , Andrea Swenson , Heena Olalde , Charlene Hafer-Macko , Justin Kwan , Lindsay Zilliox , Karen Callison , Beth DiSanzo , Kerry Naunton , Martin Bilsker , Khema Sharma , Eliana Reyes , Anne Cooley , Sara-Claude Michon , Julie Steele , Chafic Karam Karam , Manisha Chopra , Shawn Bird , Jacob Kaufman , Nichole Gallatti , Tuan Vu , Lara Katzin , Terry McClain , Brittany Harvey , Adam Hart , Kristin Huynh , Said Beydoun , Amaiak Chilingaryan , Brian Droker , Frank Lin , Akshay Shah , Anh Tran , Salma Akhter , Ali Malekniazi , Rup Tandan , Michael Hehir , Waqar Waheed , Shannon Lucy , Michael Weiss , Jane Distad , Sharon Downing , Susan Strom , Robert Lisak , Evanthia Bernitsas , Omar Khan , Shitiz Kumar Sriwastava , Alexandros Tselis , Kelly Jia , Tulio Bertorini , Thomas Arnold , Kendrick Henderson , Rekha Pillai , Ye Liu , Lauren Wheeler , Jasmine Hewlett , Mollie Vanderhook , Daniel Dicapua , Benison Keung , Aditya Kumar , Huned Patwa , Kimberly Robeson , Joan Nye , Hong Vu
The Lancet Neurology ( IF 48.0 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1474-4422(17)30369-1 James F Howard , Kimiaki Utsugisawa , Michael Benatar , Hiroyuki Murai , Richard J Barohn , Isabel Illa , Saiju Jacob , John Vissing , Ted M Burns , John T Kissel , Srikanth Muppidi , Richard J Nowak , Fanny O'Brien , Jing-Jing Wang , Renato Mantegazza , Claudio Gabriel Mazia , Miguel Wilken , Carolina Ortea , Juliet Saba , Marcelo Rugiero , Mariela Bettini , Gonzalo Vidal , Alejandra Dalila Garcia , Phillipa Lamont , Wai-Kuen Leong , Heidi Boterhoven , Beverly Fyfe , Leslie Roberts , Mahi Jasinarachchi , Natasha Willlems , Julia Wanschitz , Wolfgang Löscher , Jan De Bleecker , Guy Van den Abeele , Kathy de Koning , Katrien De Mey , Rudy Mercelis , Linda Wagemaekers , Delphine Mahieu , Philip Van Damme , Charlotte Smetcoren , Olivier Stevens , Sarah Verjans , Ann D'Hondt , Petra Tilkin , Alzira Alves de Siqueira Carvalho , Rosa Hasan , Igor Dias Brockhausen , David Feder , Daniel Ambrosio , Ana Paula Melo , Rosana Rocha , Bruno Rosa , Thabata Veiga , Luiz Augusto da Silva , Jordana Gonçalves Geraldo , Maria da Penha Morita Ananias , Erica Nogueira Coelho , Gabriel Paiva , Marina Pozo , Natalia Prando , Debora Dada Martineli Torres , Cristiani Fernanda Butinhao , Erica Coelho , Luciana Renata Cubas Volpe , Gustavo Duran , Tamires Cristina Gomes da Silva , Luiz Otavio Maia Gonçalves , Lucas Eduardo Pazetto , Luciana Souza Duca , Tomás Augusto Suriane Fialho , Maurício André Gheller Friedrich , Alexandre Guerreiro , Henrique Mohr , Maurer Pereira Martins , Daiane da Cruz Pacheco , Ana Paula Macagnan , Aline de Cassia Santos , Acary Souza Bulle Oliveira , Ana Carolina Amaral de Andrade , Marcelo Annes , Valeria Cavalcante Lino , Wladimir Pinto , Carolina Miranda , Fernanda Carrara , Iandra Souza , Angela Genge , Rami Massie , Natasha Campbell , Vera Bril , Hans Katzberg , Mehran Soltani , Eduardo Ng , Zaeem Siddiqi , Celile Phan , Derrick Blackmore , Stanislav Vohanka , Josef Bednarik , Magda Chmelikova , Marek Cierny , Stanislava Toncrova , Jana Junkerova , Barbora Kurkova , Katarina Reguliova , Olga Zapletalova , Jiri Pitha , Iveta Novakova , Michaela Tyblova , Marcela Wolfova , Ivana Jurajdova , Henning Andersen , Thomas Harbo , Lotte Vinge , Anita Mogensen , Joan Højgaard , Nanna Witting , Anne Mette Autzen , Jane Pedersen , Markus Färkkilä , Sari Atula , Anne Nyrhinen , Juha-Pekka Erälinna , Mikko Laaksonen , Olli Oksaranta , Jaana Eriksson , Tuula Harrison , Claude Desnuelle , Sabrina Sacconi , Marie-Hélène Soriani , Sonia Decressac , Julie Moutarde , Pauline Lahaut , Guilhem Solé , Gwendal Le Masson , Anne-Cécile Wielanek-Bachelet , Morgane Gaboreau , Caroline Moreau , Amy Wilson , Christophe Vial , Françoise Bouhour , Helene Gervais-Bernard , Hélène Merle , Caroline Hourquin , Arnaud Lacour , Olivier Outteryck , Patrick Vermersch , Hélène Zephir , Edouard Millois , Michel Deneve , Fabienne Deruelle , Benedikt Schoser , Stephan Wenninger , Martin Stangel , Sascha Alvermann , Stefan Gingele , Thomas Skripuletz , Kurt-Wolfram Suehs , Corinna Trebst , Karin Fricke , Sotirios Papagiannopoulos , Sevasti Bostantzopoulou , Nicholas Vlaikidis , Martha Zampaki , Nikoletta Papadopoulou , Dimos-Dimitrios Mitsikostas , Eleni Kasioti , Efstathia Mitropoulou , Despoina Charalambous , Csilla Rozsa , Melinda Horvath , Gabor Lovas , Judit Matolcsi , Gyorgyi Szabo , Brigitta Szabadosne , Laszlo Vecsei , Livia Dezsi , Edina Varga , Monika Konyane , Bella Gross , Olga Azrilin , Nelly Greenbereg , Hila Bali Kuperman , Giovanni Antonini , Matteo Garibaldi , Stefania Morino , Fernanda Troili , Antonella Di Pasquale , Alessandro Filla , Teresa Costabile , Enrico Marano , Francesco Sacca , Angela Marsil , Giorgia Puorro , Michelangelo Maestri Tassoni , Anna De Rosa , Silvia Bonanno , Carlo Antozzi , Lorenzo Maggi , Angela Campanella , Corrado Angelini , Paola Cudia , Valentina Pegoraro , Elena Pinzan , Francesca Bevilacqua , Daniele Orrico , Domenico Marco Bonifati , Amelia Evoli , Paolo Emilio Alboini , Valentina D'Amato , Raffaele Iorio , Maurizio Inghilleri , Laura Fionda , Vittorio Frasca , Elena Giacomelli , Maria Gori , Diego Lopergolo , Emanuela Onesti , Maria Gabriele , Francesco Patti , Andrea Salvatore Caramma , Silvia Messina , Ester Reggio , Cinzia Caserta , Akiyuki Uzawa , Tetsuya Kanai , Masahiro Mori , Yoko Kaneko , Akiko Kanzaki , Eri Kobayashi , Katsuhisa Masaki , Dai Matsuse , Takuya Matsushita , Taira Uehara , Misa Shimpo , Maki Jingu , Keiko Kikutake , Yumiko Nakamura , Yoshiko Sano , Yuriko Nagane , Ikuko Kamegamori , Yuko Fujii , Kazumi Futono , Tomoko Tsuda , Yuka Saito , Hidekazu Suzuki , Miyuki Morikawa , Makoto Samukawa , Sachiko Kamakura , Hirokazu Shiraishi , Teiichiro Mitazaki , Masakatsu Motomura , Akihiro Mukaino , Shunsuke Yoshimura , Shizuka Asada , Tomomi Kobashikawa , Megumi Koga , Yasuko Maeda , Kazumi Takada , Mihoko Takada Takada , Yumi Yamashita , Seiko Yoshida , Yasushi Suzuki , Tetsuya Akiyama , Koichi Narikawa , Kenichi Tsukita , Fumie Meguro , Yusuke Fukuda , Miwako Sato , Hidenori Matsuo , Takayasu Fukudome , Yuichiro Gondo , Yasuhiro Maeda , Akiko Nagaishi , Shunya Nakane , Yoshinori Okubo , Meinoshin Okumura , Soichiro Funaka , Tomohiro Kawamura , Masayuki Makamori , Masanori Takahashi , Tomoya Hasuike , Eriko Higuchi , Hisako Kobayashi , Kaori Osakada , Namie Taichi , Emiko Tsuda , Takashi Hayashi , Shin Hisahara , Tomihiro Imai , Jun Kawamata , Takashi Murahara , Masaki Saitoh , Shun Shimohama , Shuichiro Suzuki , Daisuke Yamamoto , Shingo Konno , Tomomi Imamura , Masashi Inoue , Mayumi Murata , Hiroshi Nakazora , Ritsu Nakayama , Yasuko Ikeda , Miki Ogawa , Maoko Shirane , Takashi Kanda , Motoharu Kawai , Michiaki Koga , Junichi Ogasawara , Masatoshi Omoto , Yasuteru Sano , Hideki Arima , Sachie Fukui , Shigemi Shimose , Hirokazu Shinozaki , Masanori Watanabe , Chieko Yoshikawa , Anneke van der Kooi , Marianne de Visser , Tamar Gibson , Jos Maessen , Marc de Baets , Catherine Faber , Maria Johanna Keijzers , Monique Miesen , Anna Kostera-Pruszczyk , Anna Kaminska , Byung-Jo Kim , Chang Nyoung Lee , Yong Seo Koo , Hung Youl Seok , Hoo Nam Kang , HyeJin Ra , Byoung Joon Kim , Eun Bin Cho , HyeLim Lee , Ju-Hong Min , Jinmyoung Seok , Da Yoon Koh , JuYoung Kwon , JiEun Lee , SangAe Park , Yoon-Ho Hong , Jae-Sung Lim , MiRi Kim , Seung Min Kim , Yool-hee Kim , Hyung Seok Lee , Ha Young Shin , Eun Bi Hwang , MiJu Shin , Denis Sazonov , Asya Yarmoschuk , Larisa Babenko , Nadezhda Malkova , Anna Melnikova , Denis Korobko , Evgeniya Kosykh , Dmitry Pokhabov , Yulia Nesterova , Vladislav Abramov , Victor Balyazin , Carlos Casasnovas Pons , Maria Alberti Aguilo , Christian Homedes-Pedret , Natalia Julia Palacios , Ana Lazaro , Exuperio Diez Tejedor , Mireya Fernandez-Fournier , Pedro Lopez Ruiz , Francisco Javier Rodriguez de Rivera , Maria Salvado Figueras , Josep Gamez , Maria Salvado , Elena Cortes Vicente , Jordi Diaz-Manera , Luis Querol Gutierrez , Ricardo Rojas Garcia , Nuria Vidal , Elisabet Arribas-Ibar , Fredrik Piehl , Albert Hietala , Lena Bjarbo , Christopher Lindberg , Daniel Jons , Blanka Andersson , Ihsan Sengun , Pinar Ozcelik , Celal Tuga , Muzeyyen Ugur , Cavit Boz , Didem Altiparmak , Sibel Gazioglu , Cigdem Ozen Aydin , Sevim Erdem-Ozdamar , Can Ebru Bekircan-Kurt , Ezgi Yilmaz , Nazire Pinar Acar , Yagmur Caliskan , Husnu Efendi , Seda Aydinlik , Hakan Cavus , Cansu Semiz , Ozlem Tun , Murat Terzi , Baki Dogan , Musa Kazim Onar , Sedat Sen , Tugce Kirbas Cavdar , Fiona Norwood , Aikaterini Dimitriou , Jakit Gollogly , Mohamed Mahdi-Rogers , Arshira Seddigh , Gal Maier , Faisal Sohail , Sivakumar Sathasivam , Heike Arndt , Debbie Davies , Dave Watling , Michael Rivner , J. Edward Hartmann , Brandy Quarles , Nicole Smalley , Anthony Amato , Thomas Cochrane , Mohammed Salajegheh , Kristen Roe , Katherine Amato , Shirli Toska , Gil Wolfe , Nicholas Silvestri , Kara Patrick , Karen Zakalik , Jonathan Katz , Robert Miller , Marguerite Engel , Elena Bravver , Benjamin Brooks , Sarah Plevka , Maryanne Burdette , Mohammad Sanjak , Megan Kramer , Joanne Nemeth , Clara Schommer , Vern Juel , Jeffrey Guptill , Lisa Hobson-Webb , Kate Beck , Donna Carnes , John Loor , Amanda Anderson , Dale Lange , Eliz Agopian , Jonathan Goldstein , Erin Manning , Lindsay Kaplan , Shara Holzberg , Nicole Kassebaum , Robert Pascuzzi , Cynthia Bodkin , John Kincaid , Riley Snook , Sandra Guinrich , Angela Micheels , Vinay Chaudhry , Andrea Corse , Betsy Mosmiller , Doreen Ho , Jayashri Srinivasan , Michael Vytopil , Nicholas Ventura , Stephanie Scala , Cynthia Carter , Craig Donahue , Carol Herbert , Elaine Weiner , Jonathan McKinnon , Laura Haar , Naya McKinnon , Karan Alcon , Kevin Daniels , Nadia Sattar , Dennis Jeffery , Kaitlyn McKenna , Amanda Guidon , William David , Christina Dheel , Mark Levine-Weinberg , Catherine Nigro , Ericka Simpson , Stanley H Appel , Eugene Lai , Luis Lay, Jr , Milvia Pleitez , Sharon Halton , Casey Faigle , Lisa Thompson , Mark Sivak , Susan Shin , Joan Bratton , Daniel Jacobs , Gavin Brown , Ibrez Bandukwala , Morris Brown , Jennifer Kane , Ira Blount , Miriam Freimer , J. Chad Hoyle , Julie Agriesti , Julie Khoury , Tessa Marburger , Harpreet Kaur , Diana Dimitrova , Michelle Mellion , George Sachs , Brigid Crabtree , Roseann Keo , Ele Kim Perez , Sandra Taber , James Gilchrist , Angela Andoin , Taylor Darnell , Neelam Goyal , Sarada Sakamuri , Yuen T So , Lesly Welsh Welsh , Ratna Bhavaraju-Sanka , Alejandro Tobon Gonzalez , Floyd Jones , Amy Saklad , Sharon Nations , Jaya Trivedi , Steve Hopkins , Mohamed Kazamel , Mohammad Alsharabati , Liang Lu , Sandi Mumfrey-Thomas , Amy Woodall , David Richman , Janelle Butters , Molly Lindsay , Tahseen Mozaffar , Tiyonnoh Cash , Namita Goyal , Gulmohor Roy , Veena Mathew , Fatima Maqsood , Brian Minton , H. James Jones , Jeffrey Rosenfeld , Rebekah Garcia , Sonia Garcia , Laura Echevarria , Michael Pulley , Shachie Aranke , Alan Ross Berger , Jaimin Shah , Yasmeen Shabbir , Lisa Smith , Mary Varghese , Laurie Gutmann , Ludwig Gutmann , Andrea Swenson , Heena Olalde , Charlene Hafer-Macko , Justin Kwan , Lindsay Zilliox , Karen Callison , Beth DiSanzo , Kerry Naunton , Martin Bilsker , Khema Sharma , Eliana Reyes , Anne Cooley , Sara-Claude Michon , Julie Steele , Chafic Karam Karam , Manisha Chopra , Shawn Bird , Jacob Kaufman , Nichole Gallatti , Tuan Vu , Lara Katzin , Terry McClain , Brittany Harvey , Adam Hart , Kristin Huynh , Said Beydoun , Amaiak Chilingaryan , Brian Droker , Frank Lin , Akshay Shah , Anh Tran , Salma Akhter , Ali Malekniazi , Rup Tandan , Michael Hehir , Waqar Waheed , Shannon Lucy , Michael Weiss , Jane Distad , Sharon Downing , Susan Strom , Robert Lisak , Evanthia Bernitsas , Omar Khan , Shitiz Kumar Sriwastava , Alexandros Tselis , Kelly Jia , Tulio Bertorini , Thomas Arnold , Kendrick Henderson , Rekha Pillai , Ye Liu , Lauren Wheeler , Jasmine Hewlett , Mollie Vanderhook , Daniel Dicapua , Benison Keung , Aditya Kumar , Huned Patwa , Kimberly Robeson , Joan Nye , Hong Vu
|
BACKGROUND
Complement is likely to have a role in refractory generalised myasthenia gravis, but no approved therapies specifically target this system. Results from a phase 2 study suggested that eculizumab, a terminal complement inhibitor, produced clinically meaningful improvements in patients with anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis. We further assessed the efficacy and safety of eculizumab in this patient population in a phase 3 trial. METHODS
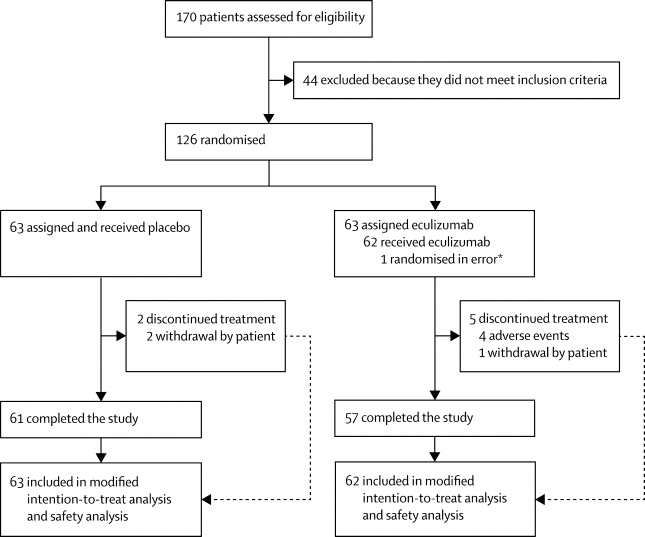
We did a phase 3, randomised, double-blind, placebo-controlled, multicentre study (REGAIN) in 76 hospitals and specialised clinics in 17 countries across North America, Latin America, Europe, and Asia. Eligible patients were aged at least 18 years, with a Myasthenia Gravis-Activities of Daily Living (MG-ADL) score of 6 or more, Myasthenia Gravis Foundation of America (MGFA) class II-IV disease, vaccination against Neisseria meningitides, and previous treatment with at least two immunosuppressive therapies or one immunosuppressive therapy and chronic intravenous immunoglobulin or plasma exchange for 12 months without symptom control. Patients with a history of thymoma or thymic neoplasms, thymectomy within 12 months before screening, or use of intravenous immunoglobulin or plasma exchange within 4 weeks before randomisation, or rituximab within 6 months before screening, were excluded. We randomly assigned participants (1:1) to either intravenous eculizumab or intravenous matched placebo for 26 weeks. Dosing for eculizumab was 900 mg on day 1 and at weeks 1, 2, and 3; 1200 mg at week 4; and 1200 mg given every second week thereafter as maintenance dosing. Randomisation was done centrally with an interactive voice or web-response system with patients stratified to one of four groups based on MGFA disease classification. Where possible, patients were maintained on existing myasthenia gravis therapies and rescue medication was allowed at the study physician's discretion. Patients, investigators, staff, and outcome assessors were masked to treatment assignment. The primary efficacy endpoint was the change from baseline to week 26 in MG-ADL total score measured by worst-rank ANCOVA. The efficacy population set was defined as all patients randomly assigned to treatment groups who received at least one dose of study drug, had a valid baseline MG-ADL assessment, and at least one post-baseline MG-ADL assessment. The safety analyses included all randomly assigned patients who received eculizumab or placebo. This trial is registered with ClinicalTrials.gov, number NCT01997229. FINDINGS
Between April 30, 2014, and Feb 19, 2016, we randomly assigned and treated 125 patients, 62 with eculizumab and 63 with placebo. The primary analysis showed no significant difference between eculizumab and placebo (least-squares mean rank 56·6 [SEM 4·5] vs 68·3 [4·5]; rank-based treatment difference -11·7, 95% CI -24·3 to 0·96; p=0·0698). No deaths or cases of meningococcal infection occurred during the study. The most common adverse events in both groups were headache and upper respiratory tract infection (ten [16%] for both events in the eculizumab group and 12 [19%] for both in the placebo group). Myasthenia gravis exacerbations were reported by six (10%) patients in the eculizumab group and 15 (24%) in the placebo group. Six (10%) patients in the eculizumab group and 12 (19%) in the placebo group required rescue therapy. INTERPRETATION
The change in the MG-ADL score was not statistically significant between eculizumab and placebo, as measured by the worst-rank analysis. Eculizumab was well tolerated. The use of a worst-rank analytical approach proved to be an important limitation of this study since the secondary and sensitivity analyses results were inconsistent with the primary endpoint result; further research into the role of complement is needed. FUNDING
Alexion Pharmaceuticals.
中文翻译:

依库珠单抗在抗乙酰胆碱受体抗体阳性难治性全身性重症肌无力 (REGAIN) 中的安全性和有效性:3 期、随机、双盲、安慰剂对照、多中心研究
背景补体可能在难治性全身性重症肌无力中起作用,但没有批准的疗法专门针对该系统。2 期研究的结果表明,依库珠单抗(一种终末补体抑制剂)对抗乙酰胆碱受体抗体阳性的难治性全身性重症肌无力患者产生了具有临床意义的改善。我们在 3 期试验中进一步评估了依库珠单抗在该患者人群中的疗效和安全性。方法 我们在北美、拉丁美洲、欧洲和亚洲 17 个国家的 76 家医院和专科诊所进行了一项随机、双盲、安慰剂对照、多中心 3 期研究 (REGAIN)。符合条件的患者年龄至少为 18 岁,重症肌无力 - 日常生活活动 (MG-ADL) 评分为 6 或更高,美国重症肌无力基金会 (MGFA) II-IV 类疾病,接种脑膜炎奈瑟菌疫苗,之前接受过至少两种免疫抑制疗法或一种免疫抑制疗法和慢性静脉注射免疫球蛋白或血浆置换治疗 12 个月,但症状未得到控制。排除有胸腺瘤或胸腺肿瘤病史、筛选前 12 个月内接受过胸腺切除术、或随机分组前 4 周内使用静脉内免疫球蛋白或血浆置换、或筛选前 6 个月内使用利妥昔单抗的患者。我们将参与者 (1:1) 随机分配至静脉注射依库珠单抗或静脉注射匹配安慰剂 26 周。在第 1 天和第 1、2 和 3 周,依库珠单抗的剂量为 900 mg;第 4 周 1200 毫克;并且此后每两周给予 1200 mg 作为维持剂量。随机化是通过交互式语音或网络响应系统集中完成的,患者根据 MGFA 疾病分类分为四组之一。在可能的情况下,患者继续接受现有的重症肌无力疗法,并根据研究医师的判断允许使用救援药物。患者、研究人员、工作人员和结果评估员对治疗分配不知情。主要疗效终点是由最差等级 ANCOVA 测量的 MG-ADL 总分从基线到第 26 周的变化。疗效人群集定义为随机分配到接受至少一剂研究药物、具有有效基线 MG-ADL 评估和至少一项基线后 MG-ADL 评估的治疗组的所有患者。安全性分析包括所有随机分配的接受依库珠单抗或安慰剂治疗的患者。该试验已在 ClinicalTrials.gov 注册,编号 NCT01997229。结果 在 2014 年 4 月 30 日至 2016 年 2 月 19 日期间,我们随机分配并治疗了 125 名患者,其中 62 名使用依库珠单抗,63 名使用安慰剂。主要分析显示依库珠单抗和安慰剂之间没有显着差异(最小二乘平均等级 56·6 [SEM 4·5] vs 68·3 [4·5];基于等级的治疗差异 -11·7,95% CI - 24·3 到 0·96;p=0·0698)。研究期间没有发生死亡或脑膜炎球菌感染病例。两组中最常见的不良事件是头痛和上呼吸道感染(依库珠单抗组的两个事件均为 10 [16%],安慰剂组均为 12 [19%])。依库珠单抗组 6 名 (10%) 患者和安慰剂组 15 名 (24%) 患者报告了重症肌无力恶化。依库珠单抗组 6 名 (10%) 患者和安慰剂组 12 名 (19%) 患者需要抢救治疗。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。
更新日期:2017-12-01
中文翻译:

依库珠单抗在抗乙酰胆碱受体抗体阳性难治性全身性重症肌无力 (REGAIN) 中的安全性和有效性:3 期、随机、双盲、安慰剂对照、多中心研究
背景补体可能在难治性全身性重症肌无力中起作用,但没有批准的疗法专门针对该系统。2 期研究的结果表明,依库珠单抗(一种终末补体抑制剂)对抗乙酰胆碱受体抗体阳性的难治性全身性重症肌无力患者产生了具有临床意义的改善。我们在 3 期试验中进一步评估了依库珠单抗在该患者人群中的疗效和安全性。方法 我们在北美、拉丁美洲、欧洲和亚洲 17 个国家的 76 家医院和专科诊所进行了一项随机、双盲、安慰剂对照、多中心 3 期研究 (REGAIN)。符合条件的患者年龄至少为 18 岁,重症肌无力 - 日常生活活动 (MG-ADL) 评分为 6 或更高,美国重症肌无力基金会 (MGFA) II-IV 类疾病,接种脑膜炎奈瑟菌疫苗,之前接受过至少两种免疫抑制疗法或一种免疫抑制疗法和慢性静脉注射免疫球蛋白或血浆置换治疗 12 个月,但症状未得到控制。排除有胸腺瘤或胸腺肿瘤病史、筛选前 12 个月内接受过胸腺切除术、或随机分组前 4 周内使用静脉内免疫球蛋白或血浆置换、或筛选前 6 个月内使用利妥昔单抗的患者。我们将参与者 (1:1) 随机分配至静脉注射依库珠单抗或静脉注射匹配安慰剂 26 周。在第 1 天和第 1、2 和 3 周,依库珠单抗的剂量为 900 mg;第 4 周 1200 毫克;并且此后每两周给予 1200 mg 作为维持剂量。随机化是通过交互式语音或网络响应系统集中完成的,患者根据 MGFA 疾病分类分为四组之一。在可能的情况下,患者继续接受现有的重症肌无力疗法,并根据研究医师的判断允许使用救援药物。患者、研究人员、工作人员和结果评估员对治疗分配不知情。主要疗效终点是由最差等级 ANCOVA 测量的 MG-ADL 总分从基线到第 26 周的变化。疗效人群集定义为随机分配到接受至少一剂研究药物、具有有效基线 MG-ADL 评估和至少一项基线后 MG-ADL 评估的治疗组的所有患者。安全性分析包括所有随机分配的接受依库珠单抗或安慰剂治疗的患者。该试验已在 ClinicalTrials.gov 注册,编号 NCT01997229。结果 在 2014 年 4 月 30 日至 2016 年 2 月 19 日期间,我们随机分配并治疗了 125 名患者,其中 62 名使用依库珠单抗,63 名使用安慰剂。主要分析显示依库珠单抗和安慰剂之间没有显着差异(最小二乘平均等级 56·6 [SEM 4·5] vs 68·3 [4·5];基于等级的治疗差异 -11·7,95% CI - 24·3 到 0·96;p=0·0698)。研究期间没有发生死亡或脑膜炎球菌感染病例。两组中最常见的不良事件是头痛和上呼吸道感染(依库珠单抗组的两个事件均为 10 [16%],安慰剂组均为 12 [19%])。依库珠单抗组 6 名 (10%) 患者和安慰剂组 15 名 (24%) 患者报告了重症肌无力恶化。依库珠单抗组 6 名 (10%) 患者和安慰剂组 12 名 (19%) 患者需要抢救治疗。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。解释 根据最差等级分析衡量,依库珠单抗和安慰剂之间的 MG-ADL 评分变化无统计学意义。依库珠单抗耐受性良好。使用最差分析方法被证明是本研究的一个重要限制,因为次要和敏感性分析结果与主要终点结果不一致;需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。需要进一步研究补体的作用。资助 Alexion Pharmaceuticals。


























 京公网安备 11010802027423号
京公网安备 11010802027423号