Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2017-11-14 , DOI: 10.1016/s0140-6736(17)32812-x Joshua D Lee 1 , Edward V Nunes 2 , Patricia Novo 3 , Ken Bachrach 4 , Genie L Bailey 5 , Snehal Bhatt 6 , Sarah Farkas 3 , Marc Fishman 7 , Phoebe Gauthier 3 , Candace C Hodgkins 8 , Jacquie King 9 , Robert Lindblad 9 , David Liu 10 , Abigail G Matthews 9 , Jeanine May 9 , K Michelle Peavy 11 , Stephen Ross 3 , Dagmar Salazar 9 , Paul Schkolnik 12 , Dikla Shmueli-Blumberg 9 , Don Stablein 9 , Geetha Subramaniam 10 , John Rotrosen 3
The Lancet ( IF 168.9 ) Pub Date : 2017-11-14 , DOI: 10.1016/s0140-6736(17)32812-x Joshua D Lee 1 , Edward V Nunes 2 , Patricia Novo 3 , Ken Bachrach 4 , Genie L Bailey 5 , Snehal Bhatt 6 , Sarah Farkas 3 , Marc Fishman 7 , Phoebe Gauthier 3 , Candace C Hodgkins 8 , Jacquie King 9 , Robert Lindblad 9 , David Liu 10 , Abigail G Matthews 9 , Jeanine May 9 , K Michelle Peavy 11 , Stephen Ross 3 , Dagmar Salazar 9 , Paul Schkolnik 12 , Dikla Shmueli-Blumberg 9 , Don Stablein 9 , Geetha Subramaniam 10 , John Rotrosen 3
Affiliation
|
BACKGROUND
Extended-release naltrexone (XR-NTX), an opioid antagonist, and sublingual buprenorphine-naloxone (BUP-NX), a partial opioid agonist, are pharmacologically and conceptually distinct interventions to prevent opioid relapse. We aimed to estimate the difference in opioid relapse-free survival between XR-NTX and BUP-NX.
METHODS
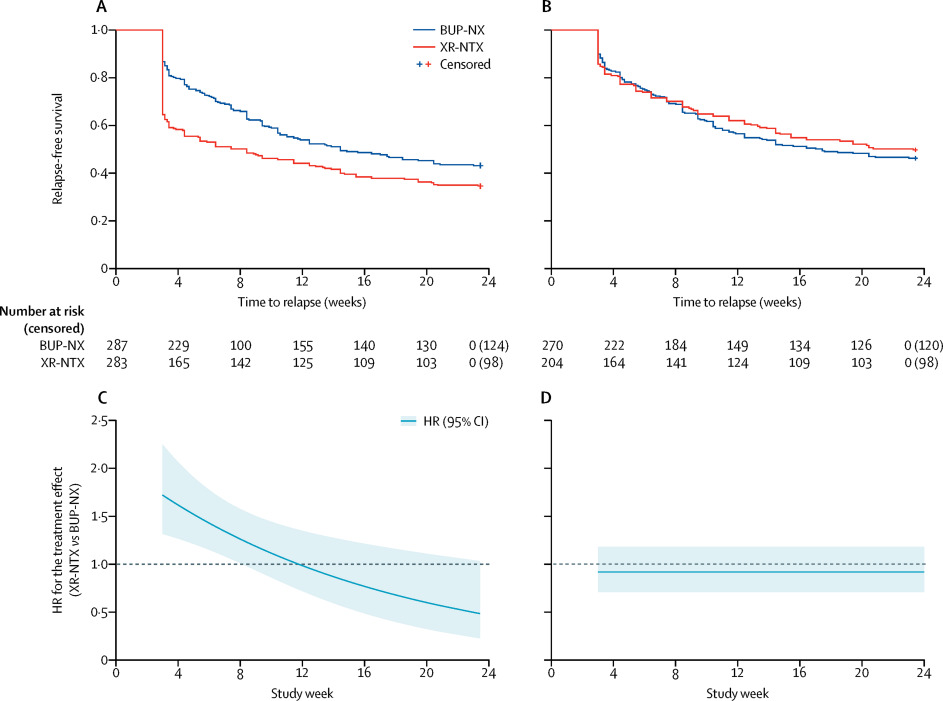
We initiated this 24 week, open-label, randomised controlled, comparative effectiveness trial at eight US community-based inpatient services and followed up participants as outpatients. Participants were 18 years or older, had Diagnostic and Statistical Manual of Mental Disorders-5 opioid use disorder, and had used non-prescribed opioids in the past 30 days. We stratified participants by treatment site and opioid use severity and used a web-based permuted block design with random equally weighted block sizes of four and six for randomisation (1:1) to receive XR-NTX or BUP-NX. XR-NTX was monthly intramuscular injections (Vivitrol; Alkermes) and BUP-NX was daily self-administered buprenorphine-naloxone sublingual film (Suboxone; Indivior). The primary outcome was opioid relapse-free survival during 24 weeks of outpatient treatment. Relapse was 4 consecutive weeks of any non-study opioid use by urine toxicology or self-report, or 7 consecutive days of self-reported use. This trial is registered with ClinicalTrials.gov, NCT02032433.
FINDINGS
Between Jan 30, 2014, and May 25, 2016, we randomly assigned 570 participants to receive XR-NTX (n=283) or BUP-NX (n=287). The last follow-up visit was Jan 31, 2017. As expected, XR-NTX had a substantial induction hurdle: fewer participants successfully initiated XR-NTX (204 [72%] of 283) than BUP-NX (270 [94%] of 287; p<0·0001). Among all participants who were randomly assigned (intention-to-treat population, n=570) 24 week relapse events were greater for XR-NTX (185 [65%] of 283) than for BUP-NX (163 [57%] of 287; hazard ratio [HR] 1·36, 95% CI 1·10-1·68), most or all of this difference accounted for by early relapse in nearly all (70 [89%] of 79) XR-NTX induction failures. Among participants successfully inducted (per-protocol population, n=474), 24 week relapse events were similar across study groups (p=0·44). Opioid-negative urine samples (p<0·0001) and opioid-abstinent days (p<0·0001) favoured BUP-NX compared with XR-NTX among the intention-to-treat population, but were similar across study groups among the per-protocol population. Self-reported opioid craving was initially less with XR-NTX than with BUP-NX (p=0·0012), then converged by week 24 (p=0·20). With the exception of mild-to-moderate XR-NTX injection site reactions, treatment-emergent adverse events including overdose did not differ between treatment groups. Five fatal overdoses occurred (two in the XR-NTX group and three in the BUP-NX group).
INTERPRETATION
In this population it is more difficult to initiate patients to XR-NTX than BUP-NX, and this negatively affected overall relapse. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
FUNDING
NIDA Clinical Trials Network.
中文翻译:

缓释纳曲酮与丁丙诺啡-纳洛酮预防阿片类药物复发的效果比较 (X:BOT):一项多中心、开放标签、随机对照试验。
背景缓释纳曲酮(XR-NTX)(一种阿片类拮抗剂)和舌下含服丁丙诺啡-纳洛酮(BUP-NX)(一种部分阿片类激动剂)在药理学和概念上是不同的预防阿片类药物复发的干预措施。我们的目的是评估 XR-NTX 和 BUP-NX 之间阿片类药物无复发生存率的差异。方法 我们在美国八个社区住院服务机构启动了这项为期 24 周的开放标签、随机对照、比较有效性试验,并对门诊患者进行随访。参与者年满 18 岁,患有《精神疾病诊断与统计手册》5 类阿片类药物使用障碍,并且在过去 30 天内使用过非处方阿片类药物。我们根据治疗部位和阿片类药物使用严重程度对参与者进行分层,并使用基于网络的排列区组设计,随机等权重区组大小为 4 和 6 进行随机化 (1:1),以接受 XR-NTX 或 BUP-NX。XR-NTX 是每月肌内注射(Vivitrol;Alkermes),BUP-NX 是每天自行施用丁丙诺啡-纳洛酮舌下膜(Suboxone;Indivior)。主要结局是 24 周门诊治疗期间阿片类药物无复发生存期。复发是指根据尿液毒理学或自我报告连续 4 周使用任何非研究阿片类药物,或连续 7 天自我报告使用。该试验已在 ClinicalTrials.gov 注册,NCT02032433。结果 2014 年 1 月 30 日至 2016 年 5 月 25 日期间,我们随机分配 570 名参与者接受 XR-NTX (n=283) 或 BUP-NX (n=287)。最后一次随访是在 2017 年 1 月 31 日。正如预期的那样,XR-NTX 存在很大的诱导障碍:成功启动 XR-NTX 的参与者(283 人中的 204 人 [72%])少于 BUP-NX(270 人 [94%]) 287;p<0·0001)。在所有随机分配的参与者中(意向治疗人群,n = 570),XR-NTX 组的 24 周复发事件(283 人中的 185 人 [65%])比 BUP-NX 组(283 人中的 163 人[57%])更大。 287;风险比 [HR] 1·36,95% CI 1·10-1·68),这种差异的大部分或全部是由几乎所有(79 例中的 70 [89%])XR-NTX 诱导的早期复发造成的失败。在成功诱导的参与者中(符合方案人群,n=474),各研究组的 24 周复发事件相似(p=0·44)。在意向治疗人群中,与 XR-NTX 相比,阿片类药物阴性尿液样本 (p<0·0001) 和阿片类药物戒断天数 (p<0·0001) 有利于 BUP-NX,但在不同研究组之间的情况相似每个协议的人口。XR-NTX 患者自我报告的阿片类药物渴望最初低于 BUP-NX 患者 (p=0·0012),然后在第 24 周时趋于一致 (p=0·20)。除了轻度至中度 XR-NTX 注射部位反应外,治疗中出现的不良事件(包括药物过量)在治疗组之间没有差异。发生了 5 起致命的用药过量事件(XR-NTX 组有 2 起,BUP-NX 组有 3 起)。解释 在这一人群中,让患者开始使用 XR-NTX 比 BUP-NX 更困难,这对总体复发产生负面影响。然而,一旦开始,这两种药物同样安全有效。未来的工作应侧重于促进 XR-NTX 的诱导并改善两种药物的治疗保留。资助 NIDA 临床试验网络。
更新日期:2018-01-26
中文翻译:

缓释纳曲酮与丁丙诺啡-纳洛酮预防阿片类药物复发的效果比较 (X:BOT):一项多中心、开放标签、随机对照试验。
背景缓释纳曲酮(XR-NTX)(一种阿片类拮抗剂)和舌下含服丁丙诺啡-纳洛酮(BUP-NX)(一种部分阿片类激动剂)在药理学和概念上是不同的预防阿片类药物复发的干预措施。我们的目的是评估 XR-NTX 和 BUP-NX 之间阿片类药物无复发生存率的差异。方法 我们在美国八个社区住院服务机构启动了这项为期 24 周的开放标签、随机对照、比较有效性试验,并对门诊患者进行随访。参与者年满 18 岁,患有《精神疾病诊断与统计手册》5 类阿片类药物使用障碍,并且在过去 30 天内使用过非处方阿片类药物。我们根据治疗部位和阿片类药物使用严重程度对参与者进行分层,并使用基于网络的排列区组设计,随机等权重区组大小为 4 和 6 进行随机化 (1:1),以接受 XR-NTX 或 BUP-NX。XR-NTX 是每月肌内注射(Vivitrol;Alkermes),BUP-NX 是每天自行施用丁丙诺啡-纳洛酮舌下膜(Suboxone;Indivior)。主要结局是 24 周门诊治疗期间阿片类药物无复发生存期。复发是指根据尿液毒理学或自我报告连续 4 周使用任何非研究阿片类药物,或连续 7 天自我报告使用。该试验已在 ClinicalTrials.gov 注册,NCT02032433。结果 2014 年 1 月 30 日至 2016 年 5 月 25 日期间,我们随机分配 570 名参与者接受 XR-NTX (n=283) 或 BUP-NX (n=287)。最后一次随访是在 2017 年 1 月 31 日。正如预期的那样,XR-NTX 存在很大的诱导障碍:成功启动 XR-NTX 的参与者(283 人中的 204 人 [72%])少于 BUP-NX(270 人 [94%]) 287;p<0·0001)。在所有随机分配的参与者中(意向治疗人群,n = 570),XR-NTX 组的 24 周复发事件(283 人中的 185 人 [65%])比 BUP-NX 组(283 人中的 163 人[57%])更大。 287;风险比 [HR] 1·36,95% CI 1·10-1·68),这种差异的大部分或全部是由几乎所有(79 例中的 70 [89%])XR-NTX 诱导的早期复发造成的失败。在成功诱导的参与者中(符合方案人群,n=474),各研究组的 24 周复发事件相似(p=0·44)。在意向治疗人群中,与 XR-NTX 相比,阿片类药物阴性尿液样本 (p<0·0001) 和阿片类药物戒断天数 (p<0·0001) 有利于 BUP-NX,但在不同研究组之间的情况相似每个协议的人口。XR-NTX 患者自我报告的阿片类药物渴望最初低于 BUP-NX 患者 (p=0·0012),然后在第 24 周时趋于一致 (p=0·20)。除了轻度至中度 XR-NTX 注射部位反应外,治疗中出现的不良事件(包括药物过量)在治疗组之间没有差异。发生了 5 起致命的用药过量事件(XR-NTX 组有 2 起,BUP-NX 组有 3 起)。解释 在这一人群中,让患者开始使用 XR-NTX 比 BUP-NX 更困难,这对总体复发产生负面影响。然而,一旦开始,这两种药物同样安全有效。未来的工作应侧重于促进 XR-NTX 的诱导并改善两种药物的治疗保留。资助 NIDA 临床试验网络。



























 京公网安备 11010802027423号
京公网安备 11010802027423号