当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A biomaterial screening approach reveals microenvironmental mechanisms of drug resistance
Integrative Biology ( IF 2.5 ) Pub Date : 2017-11-21 , DOI: 10.1039/c7ib00128b Alyssa D Schwartz 1 , Lauren E Barney , Lauren E Jansen , Thuy V Nguyen , Christopher L Hall , Aaron S Meyer , Shelly R Peyton
Integrative Biology ( IF 2.5 ) Pub Date : 2017-11-21 , DOI: 10.1039/c7ib00128b Alyssa D Schwartz 1 , Lauren E Barney , Lauren E Jansen , Thuy V Nguyen , Christopher L Hall , Aaron S Meyer , Shelly R Peyton
Affiliation
|
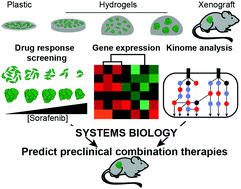
Traditional drug screening methods lack features of the tumor microenvironment that contribute to resistance. Most studies examine cell response in a single biomaterial platform in depth, leaving a gap in understanding how extracellular signals such as stiffness, dimensionality, and cell–cell contacts act independently or are integrated within a cell to affect either drug sensitivity or resistance. This is critically important, as adaptive resistance is mediated, at least in part, by the extracellular matrix (ECM) of the tumor microenvironment. We developed an approach to screen drug responses in cells cultured on 2D and in 3D biomaterial environments to explore how key features of ECM mediate drug response. This approach uncovered that cells on 2D hydrogels and spheroids encapsulated in 3D hydrogels were less responsive to receptor tyrosine kinase (RTK)-targeting drugs sorafenib and lapatinib, but not cytotoxic drugs, compared to single cells in hydrogels and cells on plastic. We found that transcriptomic differences between these in vitro models and tumor xenografts did not reveal mechanisms of ECM-mediated resistance to sorafenib. However, a systems biology analysis of phospho-kinome data uncovered that variation in MEK phosphorylation was associated with RTK-targeted drug resistance. Using sorafenib as a model drug, we found that co-administration with a MEK inhibitor decreased ECM-mediated resistance in vitro and reduced in vivo tumor burden compared to sorafenib alone. In sum, we provide a novel strategy for identifying and overcoming ECM-mediated resistance mechanisms by performing drug screening, phospho-kinome analysis, and systems biology across multiple biomaterial environments.
中文翻译:

生物材料筛选方法揭示耐药性的微环境机制
传统的药物筛选方法缺乏导致耐药性的肿瘤微环境特征。大多数研究深入研究了单个生物材料平台中的细胞反应,在理解细胞外信号(如刚度、维度和细胞 - 细胞接触)如何独立作用或整合到细胞内以影响药物敏感性或耐药性方面存在空白。这一点至关重要,因为适应性抵抗至少部分是由肿瘤微环境的细胞外基质 (ECM) 介导的。我们开发了一种方法来筛选在 2D 和 3D 生物材料环境中培养的细胞中的药物反应,以探索 ECM 的关键特征如何介导药物反应。这种方法发现,与水凝胶中的单细胞和塑料上的细胞相比,2D 水凝胶上的细胞和包裹在 3D 水凝胶中的球状体对受体酪氨酸激酶 (RTK) 靶向药物索拉非尼和拉帕替尼的反应较低,但对细胞毒性药物的反应较小。我们发现这些之间的转录组差异体外模型和肿瘤异种移植物没有揭示 ECM 介导的索拉非尼耐药机制。然而,磷酸激酶组数据的系统生物学分析发现 MEK 磷酸化的变化与 RTK 靶向耐药性相关。使用索拉非尼作为模型药物,我们发现与单独使用索拉非尼相比,与 MEK 抑制剂共同给药可降低体外ECM 介导的耐药性并降低体内肿瘤负荷。总之,我们提供了一种通过跨多种生物材料环境进行药物筛选、磷酸激酶组分析和系统生物学来识别和克服 ECM 介导的耐药机制的新策略。
更新日期:2017-11-21
中文翻译:

生物材料筛选方法揭示耐药性的微环境机制
传统的药物筛选方法缺乏导致耐药性的肿瘤微环境特征。大多数研究深入研究了单个生物材料平台中的细胞反应,在理解细胞外信号(如刚度、维度和细胞 - 细胞接触)如何独立作用或整合到细胞内以影响药物敏感性或耐药性方面存在空白。这一点至关重要,因为适应性抵抗至少部分是由肿瘤微环境的细胞外基质 (ECM) 介导的。我们开发了一种方法来筛选在 2D 和 3D 生物材料环境中培养的细胞中的药物反应,以探索 ECM 的关键特征如何介导药物反应。这种方法发现,与水凝胶中的单细胞和塑料上的细胞相比,2D 水凝胶上的细胞和包裹在 3D 水凝胶中的球状体对受体酪氨酸激酶 (RTK) 靶向药物索拉非尼和拉帕替尼的反应较低,但对细胞毒性药物的反应较小。我们发现这些之间的转录组差异体外模型和肿瘤异种移植物没有揭示 ECM 介导的索拉非尼耐药机制。然而,磷酸激酶组数据的系统生物学分析发现 MEK 磷酸化的变化与 RTK 靶向耐药性相关。使用索拉非尼作为模型药物,我们发现与单独使用索拉非尼相比,与 MEK 抑制剂共同给药可降低体外ECM 介导的耐药性并降低体内肿瘤负荷。总之,我们提供了一种通过跨多种生物材料环境进行药物筛选、磷酸激酶组分析和系统生物学来识别和克服 ECM 介导的耐药机制的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号