当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In silico analyses of essential interactions of iminosugars with the Hex A active site and evaluation of their pharmacological chaperone effects for Tay–Sachs disease
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1039/c7ob02281f Atsushi Kato 1, 2, 3, 4 , Izumi Nakagome 4, 5, 6, 7 , Shinpei Nakagawa 1, 2, 3, 4 , Kyoko Kinami 1, 2, 3, 4 , Isao Adachi 1, 2, 3, 4 , Sarah F. Jenkinson 8, 9, 10, 11, 12 , Jérôme Désiré 13, 14, 15, 16, 17 , Yves Blériot 13, 14, 15, 16, 17 , Robert J. Nash 12, 18, 19, 20, 21 , George W. J. Fleet 8, 9, 10, 11, 12 , Shuichi Hirono 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1039/c7ob02281f Atsushi Kato 1, 2, 3, 4 , Izumi Nakagome 4, 5, 6, 7 , Shinpei Nakagawa 1, 2, 3, 4 , Kyoko Kinami 1, 2, 3, 4 , Isao Adachi 1, 2, 3, 4 , Sarah F. Jenkinson 8, 9, 10, 11, 12 , Jérôme Désiré 13, 14, 15, 16, 17 , Yves Blériot 13, 14, 15, 16, 17 , Robert J. Nash 12, 18, 19, 20, 21 , George W. J. Fleet 8, 9, 10, 11, 12 , Shuichi Hirono 4, 5, 6, 7
Affiliation
|
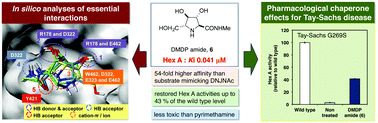
The affinity of a series of iminosugar-based inhibitors exhibiting various ring sizes toward Hex A and their essential interactions with the enzyme active site were investigated. All the Hex A-inhibiting iminosugars tested formed hydrogen bonds with Arg178, Asp322, Tyr421 and Glu462 and had the favorable cation–π interaction with Trp460. Among them, DMDP amide (6) proved to be the most potent competitive inhibitor with a Ki value of 0.041 μM. We analyzed the dynamic properties of both DMDP amide (6) and DNJNAc (1) in aqueous solution using molecular dynamics (MD) calculations; the distance of the interaction between Asp322 and 3-OH and Glu323 and 6-OH was important for stable interactions with Hex A, reducing fluctuations in the plasticity of the active site. DMDP amide (6) dose-dependently increased intracellular Hex A activity in the G269S mutant cells and restored Hex A activity up to approximately 43% of the wild type level; this effect clearly exceeded the border line treatment for Tay–Sachs disease, which is regarded as 10–15% of the wild type level. This is a significantly greater effect than that of pyrimethamine, which is currently in Phase 2 clinical trials. DMDP amide (6), therefore, represents a new promising pharmacological chaperone candidate for the treatment of Tay–Sachs disease.
中文翻译:

在计算机模拟中分析亚氨基糖与Hex A活性位点的基本相互作用并评估其对Tay-Sachs病的药理伴侣作用
研究了一系列对十六进制A表现出不同环大小的基于亚氨基糖的抑制剂的亲和力及其与酶活性位点的基本相互作用。测试的所有抑制Hex A的亚氨基糖均与Arg178,Asp322,Tyr421和Glu462形成氢键,并与Trp460具有良好的阳离子-π相互作用。其中,DMDP酰胺(6)被证明是最有效的竞争性抑制剂,K i值为0.041μM。我们分析了DMDP酰胺(6)和DNJNAc(1)的动态特性。)在水溶液中使用分子动力学(MD)计算;Asp322与3-OH和Glu323与6-OH之间相互作用的距离对于与Hex A稳定相互作用,减少活性部位可塑性的波动很重要。DMDP酰胺(6)剂量依赖性地增加G269S突变细胞中的细胞内Hex A活性,并使Hex A活性恢复至野生型水平的约43%。这种作用明显超出了针对Tay-Sachs病的界线治疗方法,后者被认为是野生型水平的10-15%。这比目前正在2期临床试验中的乙胺嘧啶的效果要大得多。DMDP酰胺(6)因此代表了一种新的有前途的药理伴侣分子疗法,可用于治疗Tay-Sachs病。
更新日期:2017-11-15
中文翻译:

在计算机模拟中分析亚氨基糖与Hex A活性位点的基本相互作用并评估其对Tay-Sachs病的药理伴侣作用
研究了一系列对十六进制A表现出不同环大小的基于亚氨基糖的抑制剂的亲和力及其与酶活性位点的基本相互作用。测试的所有抑制Hex A的亚氨基糖均与Arg178,Asp322,Tyr421和Glu462形成氢键,并与Trp460具有良好的阳离子-π相互作用。其中,DMDP酰胺(6)被证明是最有效的竞争性抑制剂,K i值为0.041μM。我们分析了DMDP酰胺(6)和DNJNAc(1)的动态特性。)在水溶液中使用分子动力学(MD)计算;Asp322与3-OH和Glu323与6-OH之间相互作用的距离对于与Hex A稳定相互作用,减少活性部位可塑性的波动很重要。DMDP酰胺(6)剂量依赖性地增加G269S突变细胞中的细胞内Hex A活性,并使Hex A活性恢复至野生型水平的约43%。这种作用明显超出了针对Tay-Sachs病的界线治疗方法,后者被认为是野生型水平的10-15%。这比目前正在2期临床试验中的乙胺嘧啶的效果要大得多。DMDP酰胺(6)因此代表了一种新的有前途的药理伴侣分子疗法,可用于治疗Tay-Sachs病。


























 京公网安备 11010802027423号
京公网安备 11010802027423号