当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Na-promoted Ni/ZrO2 dry reforming catalyst with high efficiency: details of Na2O–ZrO2–Ni interaction controlling activity and coke formation
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1039/c7cy01011g M. Németh 1, 2, 3, 4 , D. Srankó 1, 2, 3, 4 , J. Károlyi 1, 2, 3, 4 , F. Somodi 1, 2, 3, 4 , Z. Schay 1, 2, 3, 4 , G. Sáfrán 2, 3, 4, 5 , I. Sajó 4, 6, 7, 8 , A. Horváth 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1039/c7cy01011g M. Németh 1, 2, 3, 4 , D. Srankó 1, 2, 3, 4 , J. Károlyi 1, 2, 3, 4 , F. Somodi 1, 2, 3, 4 , Z. Schay 1, 2, 3, 4 , G. Sáfrán 2, 3, 4, 5 , I. Sajó 4, 6, 7, 8 , A. Horváth 1, 2, 3, 4
Affiliation
|
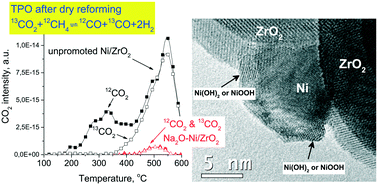
Herein, a 0.6 wt% Na-promoted 3% Ni/ZrO2 dry reforming catalyst and its unpromoted counterpart are discussed in detail. Structural investigations were carried out using TEM, TPR, XRD, XPS, and CO pulse chemisorption followed by TPD and DRIFTS methods. In the presence of a dry reforming mixture, bidentate carbonates were detected on Na-promoted Ni/ZrO2, while on Ni/ZrO2 effective hydrogenation by metallic Ni converted bicarbonates to formate species. In continuous flow atmospheric catalytic tests in a high excess of methane, a reactive-type coke was formed on the promoted sample, which did not cause significant deactivation. Temperature ramped 13CO2 isotope labeled dry reforming experiments in a closed loop sub-atmospheric circulation system revealed 13CH4 formation on Ni/ZrO2, while in the case of the promoted catalyst methanation was retarded until the complete consumption of oxidants (from 13CO2). In isothermal experiments in the same circulation system carbon monoxide disproportionation was observed on Ni/ZrO2 leaving carbon on Ni, besides the coke formed from the CH4 source, while on the promoted catalyst carbonaceous deposit under the same conditions did not form from CH4. The superb catalytic properties of Na-promoted Ni/ZrO2 are explained by a proposed catalytic cycle compiling the dynamic participation (formation and decomposition) of the surface Na2CO3 or NaHCO3 species surrounding the NiOxHy active sites on a ZrO2 support that is able to accommodate the labile Na2O promoter capturing and releasing CO2 oxidant.
中文翻译:

Na高效促进的Ni / ZrO 2干重整催化剂:Na 2 O–ZrO 2 –Ni相互作用控制活性和焦炭形成的详细信息
在本文中,详细讨论了0.6wt%Na-促进的3%Ni / ZrO 2干重整催化剂及其未促进的对应物。结构研究使用TEM,TPR,XRD,XPS和CO脉冲化学吸附,然后采用TPD和DRIFTS方法进行。在干燥重整混合物的存在下,在Na促进的Ni / ZrO 2上检测到双齿碳酸盐,而在Ni / ZrO 2上通过金属Ni转化的碳酸氢盐有效地氢化成甲酸盐。在大量过量甲烷的连续流大气催化试验中,在助燃样品上形成了反应型焦炭,但并未引起明显的失活。温度升高13 CO 2同位素标记的干重整实验在闭环低于大气压的循环系统中揭示了在Ni / ZrO 2上形成13 CH 4,而在催化剂促进的情况下,甲烷化被延迟直到氧化剂完全消耗(从13 CO 2开始)。在相同循环系统的等温实验中,除了由CH 4生成的焦炭外,在Ni / ZrO 2上还观察到一氧化碳歧化,而在Ni上留下了碳,而在促进的催化剂上,在相同条件下并未从CH 4上形成碳质沉积物。。Na促进的Ni / ZrO 2的优异催化性能通过提议的催化循环来解释,该催化循环汇编了能够容纳不稳定的Na 2 O的ZrO 2载体上NiO x H y活性位周围的表面Na 2 CO 3或NaHCO 3物种的动态参与(形成和分解)。促进剂捕获并释放CO 2氧化剂。
更新日期:2017-11-14
中文翻译:

Na高效促进的Ni / ZrO 2干重整催化剂:Na 2 O–ZrO 2 –Ni相互作用控制活性和焦炭形成的详细信息
在本文中,详细讨论了0.6wt%Na-促进的3%Ni / ZrO 2干重整催化剂及其未促进的对应物。结构研究使用TEM,TPR,XRD,XPS和CO脉冲化学吸附,然后采用TPD和DRIFTS方法进行。在干燥重整混合物的存在下,在Na促进的Ni / ZrO 2上检测到双齿碳酸盐,而在Ni / ZrO 2上通过金属Ni转化的碳酸氢盐有效地氢化成甲酸盐。在大量过量甲烷的连续流大气催化试验中,在助燃样品上形成了反应型焦炭,但并未引起明显的失活。温度升高13 CO 2同位素标记的干重整实验在闭环低于大气压的循环系统中揭示了在Ni / ZrO 2上形成13 CH 4,而在催化剂促进的情况下,甲烷化被延迟直到氧化剂完全消耗(从13 CO 2开始)。在相同循环系统的等温实验中,除了由CH 4生成的焦炭外,在Ni / ZrO 2上还观察到一氧化碳歧化,而在Ni上留下了碳,而在促进的催化剂上,在相同条件下并未从CH 4上形成碳质沉积物。。Na促进的Ni / ZrO 2的优异催化性能通过提议的催化循环来解释,该催化循环汇编了能够容纳不稳定的Na 2 O的ZrO 2载体上NiO x H y活性位周围的表面Na 2 CO 3或NaHCO 3物种的动态参与(形成和分解)。促进剂捕获并释放CO 2氧化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号