当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogenolysis of alkanes: reactions of n-butane on Ru/zeolite catalysts
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1039/c7cy00677b Geoffrey C. Bond 1, 2, 3, 4 , Juan J. Garcia 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1039/c7cy00677b Geoffrey C. Bond 1, 2, 3, 4 , Juan J. Garcia 1, 2, 3, 4
Affiliation
|
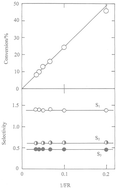
Ru/13X zeolite catalysts containing 0.6, 2 and 5.7% Ru prepared by reduction or decomposition of the hexammine complex are active and stable for n-butane hydrogenolysis between 400 and 460 K, and show similar activation energies (150–160 kJ mol−1) and orders of reaction (−1.5 to −2 in H2; 0.5–0.65 in n-butane); product selectivities show weak dependence on temperature and H2 pressure, with preference for terminal bond scission. Oxidising either the decomposed or the reduced catalysts gave a marked increase in activity, a lower activation energy (120–130 kJ mol−1), higher orders of reaction in H2 (∼−0.9), lower orders in n-butane (∼0.2) and product selectivities that showed greater change with temperature and H2 pressure; terminal bond scission was >90%. The change in character with oxidation was attributed to weaker H2 chemisorption and a higher concentration of partially dehydrogenated C4 species; the active phase may consist of very small particles of RuO2 confined within the supercages of the zeolite.
中文翻译:

烷烃的氢解:正丁烷在Ru /沸石催化剂上的反应
通过还原或分解六胺配合物制备的Ru / 13X沸石催化剂,含0.6、2和5.7%的Ru,对400-460 K的正丁烷氢解具有活性和稳定性,并显示出相似的活化能(150-160 kJ mol -1)和反应顺序(在H 2中为-1.5至-2 ;在正丁烷中为0.5-0.65 );产物选择性显示出对温度和H 2压力的弱依赖性,优选末端键断裂。氧化分解的或还原的催化剂可显着提高活性,活化能较低(120-130 kJ mol -1),H 2中的反应阶数较高(〜-0.9),n中的阶数较低-丁烷(〜0.2)和产物选择性随温度和H 2压力变化较大;末端键断裂> 90%。氧化特性的变化归因于H 2的化学吸附较弱和部分脱氢的C 4物种的浓度较高。活性相可以由限制在沸石超笼中的非常小的RuO 2颗粒组成。
更新日期:2017-11-14
中文翻译:

烷烃的氢解:正丁烷在Ru /沸石催化剂上的反应
通过还原或分解六胺配合物制备的Ru / 13X沸石催化剂,含0.6、2和5.7%的Ru,对400-460 K的正丁烷氢解具有活性和稳定性,并显示出相似的活化能(150-160 kJ mol -1)和反应顺序(在H 2中为-1.5至-2 ;在正丁烷中为0.5-0.65 );产物选择性显示出对温度和H 2压力的弱依赖性,优选末端键断裂。氧化分解的或还原的催化剂可显着提高活性,活化能较低(120-130 kJ mol -1),H 2中的反应阶数较高(〜-0.9),n中的阶数较低-丁烷(〜0.2)和产物选择性随温度和H 2压力变化较大;末端键断裂> 90%。氧化特性的变化归因于H 2的化学吸附较弱和部分脱氢的C 4物种的浓度较高。活性相可以由限制在沸石超笼中的非常小的RuO 2颗粒组成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号