当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New insights into evaluating catalyst activity and stability for oxygen evolution reactions in alkaline media
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7se00337d Guangfu Li 1, 2, 3, 4 , Lawrence Anderson 1, 2, 3, 4 , Yanan Chen 1, 2, 3, 4, 5 , Mu Pan 5, 6, 7, 8 , Po-Ya Abel Chuang 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7se00337d Guangfu Li 1, 2, 3, 4 , Lawrence Anderson 1, 2, 3, 4 , Yanan Chen 1, 2, 3, 4, 5 , Mu Pan 5, 6, 7, 8 , Po-Ya Abel Chuang 1, 2, 3, 4
Affiliation
|
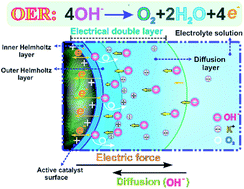
The growing development of oxygen-evolution-reaction (OER) catalysts has increased the demand for robust evaluation protocols to identify promising candidates. In this work, we successfully establish an effective experimental protocol to quantify the intrinsic performance of OER catalysts in a standard thin-film rotating-(ring)-disk-electrode system. Furthermore, a new insight into the electric double layer (EDL) microstructure is gained to illustrate all experimental observations in alkaline media. The results indicate that IrO2 has 2.6-times higher activity than NiCo2O4, due to the improved reaction kinetics, charge transfer and O2-transport occurring in the EDL. However, after 10 000 cycles of cyclic voltammetry (CV), NiCo2O4 shows much higher stability due to continuous EDL structural modifications. In particular, it is revealed that the EDL charging process, accompanied by the electrocatalytic OER, has a strong effect on catalyst–electrolyte interfacial behaviors, which, in turn, determine the accuracy of electrochemical measurement results. It is further found that the electrochemical surface area (ECSA) obtained from traditional CV measured capacitance cannot accurately reflect the actual reaction sites of various catalysts during the OER. This discrepancy exists mainly because of the EDL capacitance, which is referred to as the inner capacitance caused by less accessible active sites. Alternatively, a novel method based on in situ electrochemical impedance spectroscopy has demonstrated improved accuracy in obtaining potential-dependent ECSAs, comparable to ex situ Brunauer–Emmett–Teller surface area measurements.
中文翻译:

评估碱性介质中氧气逸出反应的催化剂活性和稳定性的新见解
氧进化反应(OER)催化剂的发展日新月异,对对确定有希望的候选物的可靠评估方案的需求不断增加。在这项工作中,我们成功地建立了一个有效的实验规程,以量化标准薄膜旋转(环)盘电极系统中OER催化剂的固有性能。此外,获得了对双电层(EDL)微观结构的新见解,以说明在碱性介质中的所有实验观察结果。结果表明,由于EDL中发生的反应动力学,电荷转移和O 2转移的改善,IrO 2的活性比NiCo 2 O 4高2.6倍。但是,经过1万次循环伏安(CV)循环后,NiCo由于连续的EDL结构修改, 2 O 4显示出更高的稳定性。特别是,发现EDL充电过程以及电催化OER会对催化剂-电解质界面行为产生强烈影响,进而决定电化学测量结果的准确性。进一步发现,从传统的CV测量电容获得的电化学表面积(ECSA)无法准确反映OER期间各种催化剂的实际反应部位。这种差异的存在主要是由于EDL电容引起的,该电容被称为内部电容,是由较少可访问的活动位点引起的。或者,一种基于原位的新方法电化学阻抗谱表明在获得依赖电位ECSAs,媲美改善的准确度易地布鲁诺-埃梅特-特勒表面积测量。
更新日期:2017-11-13
中文翻译:

评估碱性介质中氧气逸出反应的催化剂活性和稳定性的新见解
氧进化反应(OER)催化剂的发展日新月异,对对确定有希望的候选物的可靠评估方案的需求不断增加。在这项工作中,我们成功地建立了一个有效的实验规程,以量化标准薄膜旋转(环)盘电极系统中OER催化剂的固有性能。此外,获得了对双电层(EDL)微观结构的新见解,以说明在碱性介质中的所有实验观察结果。结果表明,由于EDL中发生的反应动力学,电荷转移和O 2转移的改善,IrO 2的活性比NiCo 2 O 4高2.6倍。但是,经过1万次循环伏安(CV)循环后,NiCo由于连续的EDL结构修改, 2 O 4显示出更高的稳定性。特别是,发现EDL充电过程以及电催化OER会对催化剂-电解质界面行为产生强烈影响,进而决定电化学测量结果的准确性。进一步发现,从传统的CV测量电容获得的电化学表面积(ECSA)无法准确反映OER期间各种催化剂的实际反应部位。这种差异的存在主要是由于EDL电容引起的,该电容被称为内部电容,是由较少可访问的活动位点引起的。或者,一种基于原位的新方法电化学阻抗谱表明在获得依赖电位ECSAs,媲美改善的准确度易地布鲁诺-埃梅特-特勒表面积测量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号