当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Split-Abl Kinase for Direct Activation in Cells
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1016/j.chembiol.2017.08.007 Juan E. Diaz , Charles W. Morgan , Catherine E. Minogue , Alexander S. Hebert , Joshua J. Coon , James A. Wells
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1016/j.chembiol.2017.08.007 Juan E. Diaz , Charles W. Morgan , Catherine E. Minogue , Alexander S. Hebert , Joshua J. Coon , James A. Wells
|
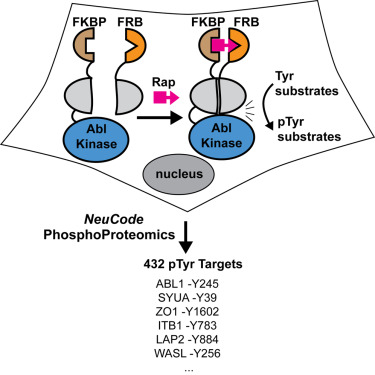
To dissect the cellular roles of individual kinases, it is useful to design tools for their selective activation. We describe the engineering of a split-cAbl kinase (sKin-Abl) that is rapidly activated in cells with rapamycin and allows temporal, dose, and compartmentalization control. Our design strategy involves an empirical screen in mammalian cells and identification of split site in the N lobe. This split site leads to complete loss of activity, which can be restored upon small-molecule-induced dimerization in cells. Remarkably, the split site is transportable to the related Src Tyr kinase and the distantly related Ser/Thr kinase, AKT, suggesting broader applications to kinases. To quantify the fold induction of phosphotyrosine (pTyr) modification, we employed quantitative proteomics, NeuCode SILAC. We identified a number of known Abl substrates, including autophosphorylation sites and novel pTyr targets, 432 pTyr sites in total. We believe that this split-kinase technology will be useful for direct activation of protein kinases in cells.
中文翻译:

一种可在细胞中直接激活的Split-Abl激酶
为了剖析单个激酶的细胞作用,设计用于其选择性激活的工具是有用的。我们描述了分裂的cAbl激酶(sKin-Abl)的工程设计,该蛋白在雷帕霉素的细胞中被迅速激活,并可以进行时间,剂量和区室控制。我们的设计策略包括在哺乳动物细胞中进行经验筛选,并鉴定N瓣中的分裂位点。该分裂位点导致活性完全丧失,可在细胞中由小分子诱导的二聚化后恢复。值得注意的是,该分裂位点可转运至相关的Src Tyr激酶和远距离相关的Ser / Thr激酶AKT,这表明该激酶具有更广泛的应用。为了量化磷酸酪氨酸(pTyr)修饰的诱导倍数,我们采用了定量蛋白质组学,NeuCode SILAC。我们鉴定了许多已知的Abl底物,包括自磷酸化位点和新型pTyr靶标,总共432 pTyr位点。我们相信,这种分裂激酶技术将对直接激活细胞中的蛋白激酶有用。
更新日期:2017-11-10
中文翻译:

一种可在细胞中直接激活的Split-Abl激酶
为了剖析单个激酶的细胞作用,设计用于其选择性激活的工具是有用的。我们描述了分裂的cAbl激酶(sKin-Abl)的工程设计,该蛋白在雷帕霉素的细胞中被迅速激活,并可以进行时间,剂量和区室控制。我们的设计策略包括在哺乳动物细胞中进行经验筛选,并鉴定N瓣中的分裂位点。该分裂位点导致活性完全丧失,可在细胞中由小分子诱导的二聚化后恢复。值得注意的是,该分裂位点可转运至相关的Src Tyr激酶和远距离相关的Ser / Thr激酶AKT,这表明该激酶具有更广泛的应用。为了量化磷酸酪氨酸(pTyr)修饰的诱导倍数,我们采用了定量蛋白质组学,NeuCode SILAC。我们鉴定了许多已知的Abl底物,包括自磷酸化位点和新型pTyr靶标,总共432 pTyr位点。我们相信,这种分裂激酶技术将对直接激活细胞中的蛋白激酶有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号