当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A domino Kornblum-DeLaMare/aza-Michael reaction of 3,6-dihydro-1,2-dioxines and application to the synthesis of the ceramide transport inhibitor (±)-HPA-12
Tetrahedron ( IF 2.1 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.tet.2017.11.010 Sarah V.A.-M. Legendre , Martyn Jevric , Julian Klepp , Christopher J. Sumby , Ben W. Greatrex
中文翻译:

3,6-二氢-1,2-二恶英的多米诺骨牌-德拉邦/氮杂-迈克尔反应及其在神经酰胺转运抑制剂(±)-HPA-12合成中的应用
更新日期:2017-11-06
Tetrahedron ( IF 2.1 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.tet.2017.11.010 Sarah V.A.-M. Legendre , Martyn Jevric , Julian Klepp , Christopher J. Sumby , Ben W. Greatrex
|
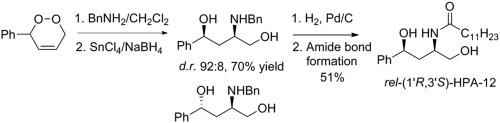
A Kornblum-DeLaMare/aza-Michael reaction of 3,6-dihydro-1,2-dioxines with primary and secondary amines has been developed which affords 4-hydroxy-3-aminoketones. The aza-Michael products were reduced using non-selective NaBH4/MeOH or diastereoselective (up to 92:8) SnCl4/NaBH4 conditions yielding (1R∗,3S∗)-3-amino-1,4-diols in up to 97% and 70% yield respectively. The major reduction product was converted in two steps to (±)-HPA-12, which is an inhibitor of the cytosolic ceramide transporting protein.
中文翻译:

3,6-二氢-1,2-二恶英的多米诺骨牌-德拉邦/氮杂-迈克尔反应及其在神经酰胺转运抑制剂(±)-HPA-12合成中的应用
已经开发了3,6-二氢-1,2-二恶英与伯胺和仲胺的Kornblum-DeLaMare /氮杂-Michael反应,其提供了4-羟基-3-氨基酮。使用非选择性NaBH 4 / MeOH或非对映选择性(最高达92:8)SnCl 4 / NaBH 4条件还原aza-Michael产物,生成(1 R ∗,3 S ∗)-3-氨基-1,4-二醇产量分别高达97%和70%。主要还原产物分两步转化为(±)-HPA-12,后者是胞质神经酰胺转运蛋白的抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号