当前位置:
X-MOL 学术
›
N. Engl. J. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-10 , DOI: 10.1056/nejmoa1709030 Jeffrey Weber , Mario Mandala , Michele Del Vecchio , Helen J. Gogas , Ana M. Arance , C. Lance Cowey , Stéphane Dalle , Michael Schenker , Vanna Chiarion-Sileni , Ivan Marquez-Rodas , Jean-Jacques Grob , Marcus O. Butler , Mark R. Middleton , Michele Maio , Victoria Atkinson , Paola Queirolo , Rene Gonzalez , Ragini R. Kudchadkar , Michael Smylie , Nicolas Meyer , Laurent Mortier , Michael B. Atkins , Georgina V. Long , Shailender Bhatia , Celeste Lebbé , Piotr Rutkowski , Kenji Yokota , Naoya Yamazaki , Tae M. Kim , Veerle de Pril , Javier Sabater , Anila Qureshi , James Larkin , Paolo A. Ascierto
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-10 , DOI: 10.1056/nejmoa1709030 Jeffrey Weber , Mario Mandala , Michele Del Vecchio , Helen J. Gogas , Ana M. Arance , C. Lance Cowey , Stéphane Dalle , Michael Schenker , Vanna Chiarion-Sileni , Ivan Marquez-Rodas , Jean-Jacques Grob , Marcus O. Butler , Mark R. Middleton , Michele Maio , Victoria Atkinson , Paola Queirolo , Rene Gonzalez , Ragini R. Kudchadkar , Michael Smylie , Nicolas Meyer , Laurent Mortier , Michael B. Atkins , Georgina V. Long , Shailender Bhatia , Celeste Lebbé , Piotr Rutkowski , Kenji Yokota , Naoya Yamazaki , Tae M. Kim , Veerle de Pril , Javier Sabater , Anila Qureshi , James Larkin , Paolo A. Ascierto
|
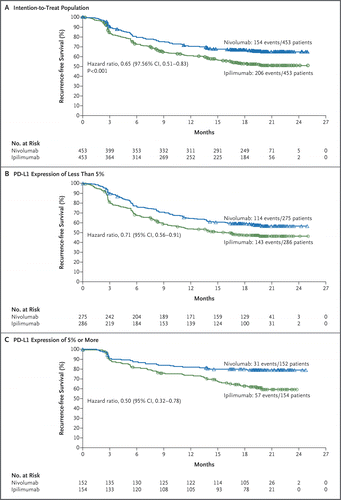
Among patients undergoing resection of stage IIIB, IIIC, or IV melanoma, adjuvant therapy with nivolumab resulted in significantly longer recurrence-free survival and a lower rate of grade 3 or 4 adverse events than adjuvant therapy with ipilimumab. (Funded by Bristol-Myers Squibb and Ono Pharmaceutical; CheckMate 238 ClinicalTrials.gov number, NCT02388906; Eudra-CT number, 2014-002351-26.)
中文翻译:

III期或IV期黑色素瘤切除术的佐剂Nivolumab与Ipilimumab的比较
在接受IIIB,IIIC或IV期黑色素瘤切除的患者中,与依普利单抗辅助治疗相比,与nivolumab辅助治疗可显着延长无复发生存期,并降低3级或4级不良事件发生率。(由Bristol-Myers Squibb和Ono Pharmaceutical资助; CheckMate 238 ClinicalTrials.gov号,NCT02388906 ; Eudra-CT号,2014-002351-26。)
更新日期:2017-11-09
中文翻译:

III期或IV期黑色素瘤切除术的佐剂Nivolumab与Ipilimumab的比较
在接受IIIB,IIIC或IV期黑色素瘤切除的患者中,与依普利单抗辅助治疗相比,与nivolumab辅助治疗可显着延长无复发生存期,并降低3级或4级不良事件发生率。(由Bristol-Myers Squibb和Ono Pharmaceutical资助; CheckMate 238 ClinicalTrials.gov号,NCT02388906 ; Eudra-CT号,2014-002351-26。)



























 京公网安备 11010802027423号
京公网安备 11010802027423号