当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ligand Substituent Effects in Manganese Pyridinophane Complexes: Implications for Oxygen-Evolving Catalysis
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02421 Song Xu 1 , Lukas Bucinsky 2 , Martin Breza 2 , J. Krzystek 3 , Chun-Hsing Chen 1 , Maren Pink 1 , Joshua Telser 4 , Jeremy M. Smith 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02421 Song Xu 1 , Lukas Bucinsky 2 , Martin Breza 2 , J. Krzystek 3 , Chun-Hsing Chen 1 , Maren Pink 1 , Joshua Telser 4 , Jeremy M. Smith 1
Affiliation
|
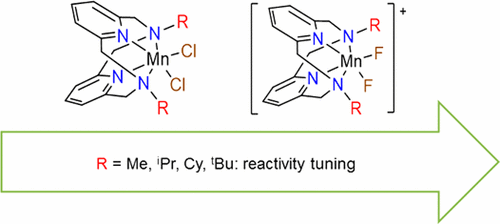
A series of Mn(II) complexes of differently substituted pyridinophane ligands, (Py2NR2)MnCl2 (R = iPr, Cy) and [(Py2NR2)MnF2](PF6) (R = iPr, Cy, tBu) are synthesized and characterized. The electrochemical properties of these complexes are investigated by cyclic voltammetry, along with those of previously reported (Py2NMe2)MnCl2 and the Mn(III) complex [(Py2NMe2)MnF2](PF6). The electronic structure of this and other Mn(III) complexes is probed experimentally and theoretically, via high-frequency and -field electron paramagnetic resonance (HFEPR) spectroscopy ab initio quantum chemical theory (QCT), respectively. These studies show that the complexes contain relatively typical six-coordinate Mn(III). The catalytic activity of these complexes toward both H2O2 disproportionation and H2O oxidation has also been investigated. The rate of H2O2 disproportionation decreases with increasing substituent size. Some of these complexes are active for electrocatalytic H2O oxidation; however this activity cannot be rationalized in terms of simple electronic or steric effects.
中文翻译:

锰吡啶鎓配合物中的配体取代基效应:对析氧催化的影响。
(Py 2 NR 2)MnCl 2(R = i Pr,Cy)和[(Py 2 NR 2)MnF 2 ](PF 6)(R = i Pr ,Cy,t Bu)被合成和表征。通过循环伏安法研究了这些络合物的电化学性质,以及先前报道的(Py 2 NMe 2)MnCl 2和Mn(III)络合物[(Py 2 NMe 2)MnF 2 ](PF 6)的电化学性质。)。分别通过高频和场电子顺磁共振(HFEPR)光谱从头计算量子化学原理(QCT),通过实验和理论方法探索了这种和其他Mn(III)配合物的电子结构。这些研究表明,该配合物包含相对典型的六坐标Mn(III)。还研究了这些配合物对H 2 O 2歧化和H 2 O氧化的催化活性。H 2 O 2歧化速率随取代基尺寸的增加而降低。这些络合物中的一些对电催化H 2具有活性O氧化;但是,就简单的电子或立体效果而言,不能合理地进行这项活动。
更新日期:2017-11-08
中文翻译:

锰吡啶鎓配合物中的配体取代基效应:对析氧催化的影响。
(Py 2 NR 2)MnCl 2(R = i Pr,Cy)和[(Py 2 NR 2)MnF 2 ](PF 6)(R = i Pr ,Cy,t Bu)被合成和表征。通过循环伏安法研究了这些络合物的电化学性质,以及先前报道的(Py 2 NMe 2)MnCl 2和Mn(III)络合物[(Py 2 NMe 2)MnF 2 ](PF 6)的电化学性质。)。分别通过高频和场电子顺磁共振(HFEPR)光谱从头计算量子化学原理(QCT),通过实验和理论方法探索了这种和其他Mn(III)配合物的电子结构。这些研究表明,该配合物包含相对典型的六坐标Mn(III)。还研究了这些配合物对H 2 O 2歧化和H 2 O氧化的催化活性。H 2 O 2歧化速率随取代基尺寸的增加而降低。这些络合物中的一些对电催化H 2具有活性O氧化;但是,就简单的电子或立体效果而言,不能合理地进行这项活动。



























 京公网安备 11010802027423号
京公网安备 11010802027423号