当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A mini-library system to investigate non-essential residues of lipid-phosphatidylserine (PS) binding peptide–peptoid hybrid PPS1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7md00372b Satya Prakash Shukla 1, 2, 3, 4 , D. Gomika Udugamasooriya 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7md00372b Satya Prakash Shukla 1, 2, 3, 4 , D. Gomika Udugamasooriya 1, 2, 3, 4, 5
Affiliation
|
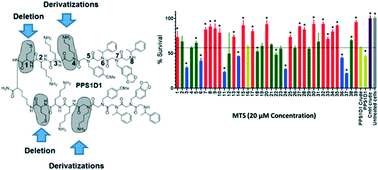
We recently identified a peptide–peptoid hybrid, PPS1, which specifically recognized lipid-phosphatidylserine (PS). PPS1 consists of distinct positively charged and hydrophobic residue-containing regions. The PPS1 monomer was inactive, but the dimeric form, PPS1D1, displayed strong cytotoxicity to lung cancer cells compared to normal cells in vitro, and reduced the tumor growth in vivo. The minimum pharmacophore of PPS1D1 showed that the first (methionine) and fourth (N-lysine) residues were not important for PPS1D1 cytotoxic activity. In this study, we further investigated these two residues, in particular the fourth residue that lies between the most important four-residue hydrophobic region and two positively charged residues, to determine whether replacements of these moieties could gain activity improvements or render PPS1D1 totally insensitive for binding recognition. The positively charged fourth residue N-lysine was replaced with substituents having varied physiochemical properties, such as aromatic-hydrophobic, aliphatic-alicyclic, heterocyclic, and negatively charged residues, developing a mini-library of 39 derivatives. The standard 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) colorimetric and/or the calcein AM cell viability assays performed on HCC4017 lung cancer cells indicated that the fourth position of PPS1D1 was insensitive to most changes, except that reversal of the negative charge significantly affected the activity. This observation may be due to the neutralization of the nearby positively charged residue that is essential for binding. In addition, shortening each monomeric sequence by eliminating the methionine at the first position did not affect the activity.
中文翻译:

一个微型库系统,用于研究脂质-磷脂酰丝氨酸(PS)结合肽-类肽杂化PPS1的非必需残基
我们最近鉴定了一种肽-类肽杂合体PPS1,它可以特异性识别脂质-磷脂酰丝氨酸(PS)。PPS1由不同的带正电和疏水性残基的区域组成。PPS1单体是无活性的,但与体外正常细胞相比,二聚体形式PPS1D1对肺癌细胞显示出强大的细胞毒性,并降低了体内肿瘤的生长。PPS1D1的最小药效基团显示第一个(甲硫氨酸)和第四个(N-赖氨酸)残基对PPS1D1的细胞毒性活性并不重要。在这项研究中,我们进一步研究了这两个残基,特别是位于最重要的四个残基疏水区域和两个带正电荷的残基之间的第四个残基,以确定这些基团的取代是否可以提高活性或使PPS1D1完全不敏感绑定识别。带正电的第四残基N-赖氨酸被具有不同理化特性的取代基所取代,例如芳香族疏水性,脂族-脂环族,杂环和带负电的残基,从而形成了39种衍生物的小型文库。标准3-(4,5-二甲基噻唑-2-基)-5-(3-羧基甲氧基苯基)-2-(4-磺基苯基)-2 H在HCC4017肺癌细胞上进行的-四唑鎓(MTS)比色法和/或钙黄绿素AM细胞生存力测定表明,PPS1D1的第四个位置对大多数变化不敏感,除了负电荷的反转显着影响了活性。该观察结果可能是由于附近的带正电的残基被结合所必需。另外,通过在第一位置消除甲硫氨酸来缩短每个单体序列不影响活性。
更新日期:2017-11-09
中文翻译:

一个微型库系统,用于研究脂质-磷脂酰丝氨酸(PS)结合肽-类肽杂化PPS1的非必需残基
我们最近鉴定了一种肽-类肽杂合体PPS1,它可以特异性识别脂质-磷脂酰丝氨酸(PS)。PPS1由不同的带正电和疏水性残基的区域组成。PPS1单体是无活性的,但与体外正常细胞相比,二聚体形式PPS1D1对肺癌细胞显示出强大的细胞毒性,并降低了体内肿瘤的生长。PPS1D1的最小药效基团显示第一个(甲硫氨酸)和第四个(N-赖氨酸)残基对PPS1D1的细胞毒性活性并不重要。在这项研究中,我们进一步研究了这两个残基,特别是位于最重要的四个残基疏水区域和两个带正电荷的残基之间的第四个残基,以确定这些基团的取代是否可以提高活性或使PPS1D1完全不敏感绑定识别。带正电的第四残基N-赖氨酸被具有不同理化特性的取代基所取代,例如芳香族疏水性,脂族-脂环族,杂环和带负电的残基,从而形成了39种衍生物的小型文库。标准3-(4,5-二甲基噻唑-2-基)-5-(3-羧基甲氧基苯基)-2-(4-磺基苯基)-2 H在HCC4017肺癌细胞上进行的-四唑鎓(MTS)比色法和/或钙黄绿素AM细胞生存力测定表明,PPS1D1的第四个位置对大多数变化不敏感,除了负电荷的反转显着影响了活性。该观察结果可能是由于附近的带正电的残基被结合所必需。另外,通过在第一位置消除甲硫氨酸来缩短每个单体序列不影响活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号