当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbon Dioxide Insertion into Group 9 and 10 Metal–Element σ Bonds
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02315 Nilay Hazari 1 , Jessica E. Heimann 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02315 Nilay Hazari 1 , Jessica E. Heimann 1
Affiliation
|
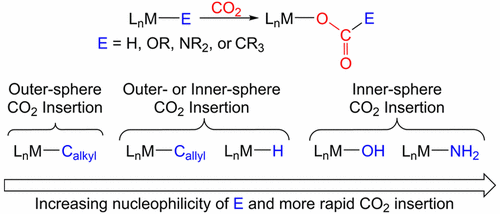
Carbon dioxide (CO2) is an appealing feedstock for the sustainable preparation of a variety of carbon-based commodity chemicals because of its high abundance, low cost, and nontoxicity. The high kinetic and thermodynamic stability of CO2, however, means that there are currently only a limited number of practical catalytic systems for the conversion of CO2 into more valuable chemicals, and continued research in this area is required. One promising approach for the eventual transformation of CO2 is to initially insert the molecule into transition-metal–element σ bonds such as M–H, M–OR, M–NR2, and M–CR3 bonds to form products of the type M–OC(O)E (E = H, OR, NR2, or CR3). CO2 insertion has been demonstrated in numerous stoichiometric reactions involving transition-metal complexes, but in cases where insertion results in the formation of strong M–O bonds, the products are often too stable to undergo further transformations. Group 9 and 10 transition-metal complexes (M = Ni, Pd, Pt, Co, Rh, or Ir) form relatively weak M–O bonds, and as a consequence, a number of group 9 and 10 transition-metal catalysts in which CO2 insertion is proposed as an elementary step in catalysis have been developed. In this Award Article, we summarize group 9 and 10 transition-metal complexes in which CO2 insertion into a metal–element σ bond to form a M–OC(O)E-type product has been observed. Mechanistic similarities and differences are highlighted by comparing CO2 insertion reactions in different types of group 9 and 10 metal–element σ bonds, and a general trend for predicting the rate-determining step of the insertion process is described based on the nucleophilicity of the element in the σ bond. Although we focus on stoichiometric reactivity, the relevance of CO2 insertion to catalytic reactions is also emphasized throughout the paper.
中文翻译:

二氧化碳插入第9和10组金属元素σ键
二氧化碳(CO 2)具有丰富,低成本和无毒的特性,因此是可持续制备多种碳基商品化学品的诱人原料。但是,CO 2的高动力学和热力学稳定性意味着,目前只有有限数量的实用催化系统可将CO 2转化为更有价值的化学物质,因此需要在这一领域进行持续的研究。最终转化CO 2的一种有前途的方法是,首先将分子插入过渡金属元素的σ键(例如M–H,M–OR,M–NR 2和M–CR 3键)中,以形成CO的产物。 M–OC(O)E类型(E = H,OR,NR 2,或CR 3)。在涉及过渡金属配合物的许多化学计量反应中都已证明了CO 2的插入,但是在插入导致形成强M-O键的情况下,产物通常太稳定而无法进行进一步的转化。第9族和第10族过渡金属配合物(M = Ni,Pd,Pt,Co,Rh或Ir)形成相对弱的M-O键,因此,许多第9族和第10族过渡金属催化剂中已提出将CO 2插入作为催化中的基本步骤。在该奖项文章中,我们总结了第9组和第10组过渡金属配合物,其中CO 2已经观察到插入金属元素的σ键以形成M–OC(O)E型产物。通过比较不同类型的第9组和第10组金属元素σ键中的CO 2插入反应,突出了机理的异同,并基于元素的亲核性描述了预测插入过程的速率决定步骤的总体趋势。在σ键中。尽管我们专注于化学计量反应性,但整篇论文还强调了CO 2插入与催化反应的相关性。
更新日期:2017-11-08
中文翻译:

二氧化碳插入第9和10组金属元素σ键
二氧化碳(CO 2)具有丰富,低成本和无毒的特性,因此是可持续制备多种碳基商品化学品的诱人原料。但是,CO 2的高动力学和热力学稳定性意味着,目前只有有限数量的实用催化系统可将CO 2转化为更有价值的化学物质,因此需要在这一领域进行持续的研究。最终转化CO 2的一种有前途的方法是,首先将分子插入过渡金属元素的σ键(例如M–H,M–OR,M–NR 2和M–CR 3键)中,以形成CO的产物。 M–OC(O)E类型(E = H,OR,NR 2,或CR 3)。在涉及过渡金属配合物的许多化学计量反应中都已证明了CO 2的插入,但是在插入导致形成强M-O键的情况下,产物通常太稳定而无法进行进一步的转化。第9族和第10族过渡金属配合物(M = Ni,Pd,Pt,Co,Rh或Ir)形成相对弱的M-O键,因此,许多第9族和第10族过渡金属催化剂中已提出将CO 2插入作为催化中的基本步骤。在该奖项文章中,我们总结了第9组和第10组过渡金属配合物,其中CO 2已经观察到插入金属元素的σ键以形成M–OC(O)E型产物。通过比较不同类型的第9组和第10组金属元素σ键中的CO 2插入反应,突出了机理的异同,并基于元素的亲核性描述了预测插入过程的速率决定步骤的总体趋势。在σ键中。尽管我们专注于化学计量反应性,但整篇论文还强调了CO 2插入与催化反应的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号