当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
O2 Activation by Metal Surfaces: Implications for Bonding and Reactivity on Heterogeneous Catalysts
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.chemrev.7b00217 Matthew M. Montemore 1, 2 , Matthijs A. van Spronsen 1 , Robert J. Madix 2 , Cynthia M. Friend 1, 2
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.chemrev.7b00217 Matthew M. Montemore 1, 2 , Matthijs A. van Spronsen 1 , Robert J. Madix 2 , Cynthia M. Friend 1, 2
Affiliation
|
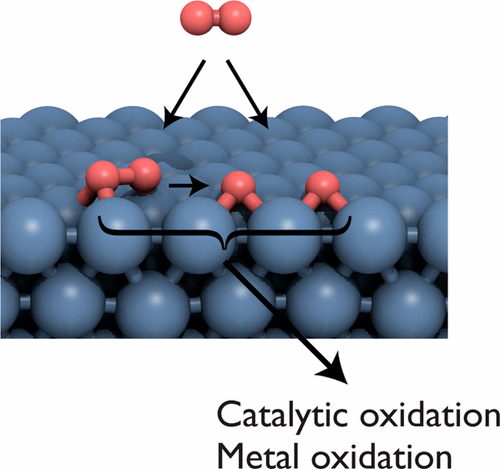
The activation of O2 on metal surfaces is a critical process for heterogeneous catalysis and materials oxidation. Fundamental studies of well-defined metal surfaces using a variety of techniques have given crucial insight into the mechanisms, energetics, and dynamics of O2 adsorption and dissociation. Here, trends in the activation of O2 on transition metal surfaces are discussed, and various O2 adsorption states are described in terms of both electronic structure and geometry. The mechanism and dynamics of O2 dissociation are also reviewed, including the importance of the spin transition. The reactivity of O2 and O toward reactant molecules is also briefly discussed in the context of catalysis. The reactivity of a surface toward O2 generally correlates with the adsorption strength of O, the tendency to oxidize, and the heat of formation of the oxide. Periodic trends can be rationalized in terms of attractive and repulsive interactions with the d-band, such that inert metals tend to feature a full d band that is low energy and has a large spatial overlap with adsorbate states. More open surfaces or undercoordinated defect sites can be much more reactive than close-packed surfaces. Reactions between O and other species tend to be more prevalent than reactions between O2 and other species, particularly on more reactive surfaces.
中文翻译:

金属表面的O 2活化:对多相催化剂上键合和反应性的影响
O 2在金属表面的活化是非均相催化和材料氧化的关键过程。使用多种技术对定义明确的金属表面进行的基础研究对O 2吸附和解离的机理,能量学和动力学提供了至关重要的见解。在此,讨论了过渡金属表面上O 2活化的趋势,并根据电子结构和几何形状描述了各种O 2吸附状态。还回顾了O 2离解的机理和动力学,包括自旋转变的重要性。O 2的反应性在催化的背景下,还简要讨论了O和O向反应物分子的转化。表面对O 2的反应性通常与O的吸附强度,氧化趋势和氧化物形成热相关。可以根据与d波段的吸引和排斥相互作用合理化周期性趋势,以使惰性金属趋向于具有低能量的完整d波段,并且与被吸附物的状态具有较大的空间重叠。与密排表面相比,更多的开放表面或欠协调的缺陷部位可能具有更大的反应性。O和其他物种之间的反应往往比O 2和其他物种之间的反应更普遍,尤其是在反应性更高的表面上。
更新日期:2017-11-08
中文翻译:

金属表面的O 2活化:对多相催化剂上键合和反应性的影响
O 2在金属表面的活化是非均相催化和材料氧化的关键过程。使用多种技术对定义明确的金属表面进行的基础研究对O 2吸附和解离的机理,能量学和动力学提供了至关重要的见解。在此,讨论了过渡金属表面上O 2活化的趋势,并根据电子结构和几何形状描述了各种O 2吸附状态。还回顾了O 2离解的机理和动力学,包括自旋转变的重要性。O 2的反应性在催化的背景下,还简要讨论了O和O向反应物分子的转化。表面对O 2的反应性通常与O的吸附强度,氧化趋势和氧化物形成热相关。可以根据与d波段的吸引和排斥相互作用合理化周期性趋势,以使惰性金属趋向于具有低能量的完整d波段,并且与被吸附物的状态具有较大的空间重叠。与密排表面相比,更多的开放表面或欠协调的缺陷部位可能具有更大的反应性。O和其他物种之间的反应往往比O 2和其他物种之间的反应更普遍,尤其是在反应性更高的表面上。


























 京公网安备 11010802027423号
京公网安备 11010802027423号