当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alkyne Benzannulation Reactions for the Synthesis of Novel Aromatic Architectures
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.accounts.7b00385 Samuel J. Hein 1, 2 , Dan Lehnherr 2 , Hasan Arslan 1, 2 , Fernando J. Uribe-Romo 2 , William R. Dichtel 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.accounts.7b00385 Samuel J. Hein 1, 2 , Dan Lehnherr 2 , Hasan Arslan 1, 2 , Fernando J. Uribe-Romo 2 , William R. Dichtel 1, 2
Affiliation
|
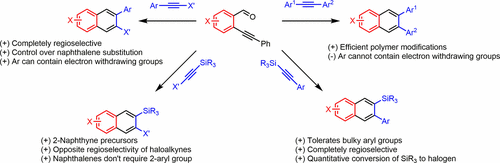
Aromatic compounds and polymers are integrated into organic field effect transistors, light-emitting diodes, photovoltaic devices, and redox-flow batteries. These compounds and materials feature increasingly complex designs, and substituents influence energy levels, bandgaps, solution conformation, and crystal packing, all of which impact performance. However, many polycyclic aromatic hydrocarbons of interest are difficult to prepare because their substitution patterns lie outside the scope of current synthetic methods, as strategies for functionalizing benzene are often unselective when applied to naphthalene or larger systems. For example, cross-coupling and nucleophilic aromatic substitution reactions rely on prefunctionalized arenes, and even directed metalation methods most often modify positions near Lewis basic sites. Similarly, electrophilic aromatic substitutions access single regioisomers under substrate control. Cycloadditions provide a convergent route to densely functionalized aromatic compounds that compliment the above methods. After surveying cycloaddition reactions that might be used to modify the conjugated backbone of poly(phenylene ethynylene)s, we discovered that the Asao–Yamamoto benzannulation reaction is notably efficient. Although this reaction had been reported a decade earlier, its scope and usefulness for synthesizing complex aromatic systems had been under-recognized. This benzannulation reaction combines substituted 2-(phenylethynyl)benzaldehydes and substituted alkynes to form 2,3-substituted naphthalenes. The reaction tolerates a variety of sterically congested alkynes, making it well-suited for accessing poly- and oligo(ortho-arylene)s and contorted hexabenzocoronenes. In many cases in which asymmetric benzaldehyde and alkyne cycloaddition partners are used, the reaction is regiospecific based on the electronic character of the alkyne substrate. Recognizing these desirable features, we broadened the substrate scope to include silyl- and halogen-substituted alkynes. Through a combined experimental and computational approach, we have elucidated mechanistic insight and key principles that govern the regioselectivity outcome of the benzannulation of structurally diverse alkynes.
中文翻译:

炔烃苯环化反应用于合成新型芳香结构
芳香族化合物和聚合物被集成到有机场效应晶体管,发光二极管,光伏器件和氧化还原液流电池中。这些化合物和材料的设计日益复杂,取代基会影响能级,带隙,溶液构象和晶体堆积,所有这些都会影响性能。然而,许多感兴趣的多环芳烃很难制备,因为它们的取代方式不在当前合成方法的范围之内,因为当将苯官能化的策略应用于萘或更大的体系时,它们往往是非选择性的。例如,交叉偶联和亲核芳族取代反应依赖于预官能化的芳烃,甚至定向金属化方法最经常会修饰Lewis碱性位点附近的位置。相似地,亲电子芳族取代在底物控制下进入单个区域异构体。环加成反应提供了一种收敛路线,从而达到了稠密官能化的芳香族化合物的要求,这是上述方法的补充。在调查了可用于修饰聚苯撑乙炔的共轭骨架的环加成反应后,我们发现麻生山本-山本苯甲酸环化反应特别有效。尽管该反应早在十年前就已被报道,但其对于合成复杂的芳族体系的范围和实用性仍未得到充分认识。该苯环化反应将取代的2-(苯基乙炔基)苯甲醛和取代的炔烃结合形成2,3-取代的萘。该反应可耐受多种空间拥塞的炔烃,使其非常适合于访问聚和寡聚(邻亚芳基)和扭曲的六苯并Coronenes。在许多情况下,使用不对称的苯甲醛和炔烃环加成伙伴,基于炔烃底物的电子特性,该反应是区域特异性的。认识到这些理想的特征,我们将底物范围扩大到包括甲硅烷基和卤素取代的炔烃。通过组合的实验和计算方法,我们阐明了机理的见解和控制结构多样的炔烃苯环化的区域选择性结果的关键原理。
更新日期:2017-11-07
中文翻译:

炔烃苯环化反应用于合成新型芳香结构
芳香族化合物和聚合物被集成到有机场效应晶体管,发光二极管,光伏器件和氧化还原液流电池中。这些化合物和材料的设计日益复杂,取代基会影响能级,带隙,溶液构象和晶体堆积,所有这些都会影响性能。然而,许多感兴趣的多环芳烃很难制备,因为它们的取代方式不在当前合成方法的范围之内,因为当将苯官能化的策略应用于萘或更大的体系时,它们往往是非选择性的。例如,交叉偶联和亲核芳族取代反应依赖于预官能化的芳烃,甚至定向金属化方法最经常会修饰Lewis碱性位点附近的位置。相似地,亲电子芳族取代在底物控制下进入单个区域异构体。环加成反应提供了一种收敛路线,从而达到了稠密官能化的芳香族化合物的要求,这是上述方法的补充。在调查了可用于修饰聚苯撑乙炔的共轭骨架的环加成反应后,我们发现麻生山本-山本苯甲酸环化反应特别有效。尽管该反应早在十年前就已被报道,但其对于合成复杂的芳族体系的范围和实用性仍未得到充分认识。该苯环化反应将取代的2-(苯基乙炔基)苯甲醛和取代的炔烃结合形成2,3-取代的萘。该反应可耐受多种空间拥塞的炔烃,使其非常适合于访问聚和寡聚(邻亚芳基)和扭曲的六苯并Coronenes。在许多情况下,使用不对称的苯甲醛和炔烃环加成伙伴,基于炔烃底物的电子特性,该反应是区域特异性的。认识到这些理想的特征,我们将底物范围扩大到包括甲硅烷基和卤素取代的炔烃。通过组合的实验和计算方法,我们阐明了机理的见解和控制结构多样的炔烃苯环化的区域选择性结果的关键原理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号