当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of the Intra- and Interlaboratory Reproducibility of a Small Scale Standardized Supersaturation and Precipitation Method
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00419 Jakob Plum 1 , Cecilie M Madsen 1, 2 , Alexandra Teleki 3 , Jan Bevernage 4 , Claudia da Costa Mathews 5 , Eva M Karlsson 6 , Sara Carlert 7 , Rene Holm 8 , Thomas Müller 9 , Wayne Matthews 10 , Alice Sayers 10 , Krista Ojala 11 , Konstantin Tsinsman 12 , Ram Lingamaneni 12 , Christel AS Bergström 3 , Thomas Rades 1 , Anette Müllertz 1, 13
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00419 Jakob Plum 1 , Cecilie M Madsen 1, 2 , Alexandra Teleki 3 , Jan Bevernage 4 , Claudia da Costa Mathews 5 , Eva M Karlsson 6 , Sara Carlert 7 , Rene Holm 8 , Thomas Müller 9 , Wayne Matthews 10 , Alice Sayers 10 , Krista Ojala 11 , Konstantin Tsinsman 12 , Ram Lingamaneni 12 , Christel AS Bergström 3 , Thomas Rades 1 , Anette Müllertz 1, 13
Affiliation
|
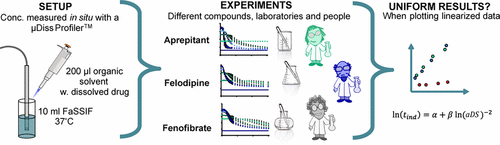
The high number of poorly water-soluble compounds in drug development has increased the need for enabling formulations to improve oral bioavailability. One frequently applied approach is to induce supersaturation at the absorptive site, e.g., the small intestine, increasing the amount of dissolved compound available for absorption. However, due to the stochastic nature of nucleation, supersaturating drug delivery systems may lead to inter- and intrapersonal variability. The ability to define a feasible range with respect to the supersaturation level is a crucial factor for a successful formulation. Therefore, an in vitro method is needed, from where the ability of a compound to supersaturate can be defined in a reproducible way. Hence, this study investigates the reproducibility of an in vitro small scale standardized supersaturation and precipitation method (SSPM). First an intralaboratory reproducibility study of felodipine was conducted, after which seven partners contributed with data for three model compounds; aprepitant, felodipine, and fenofibrate, to determine the interlaboratory reproducibility of the SSPM. The first part of the SSPM determines the apparent degrees of supersaturation (aDS) to investigate for each compound. Each partner independently determined the maximum possible aDS and induced 100, 87.5, 75, and 50% of their determined maximum possible aDS in the SSPM. The concentration–time profile of the supersaturation and following precipitation was obtained in order to determine the induction time (tind) for detectable precipitation. The data showed that the absolute values of tind and aDS were not directly comparable between partners, however, upon linearization of the data a reproducible rank ordering of the three model compounds was obtained based on the β-value, which was defined as the slope of the ln(tind) versus ln(aDS)−2 plot. Linear regression of this plot showed that aprepitant had the highest β-value, 15.1, while felodipine and fenofibrate had comparable β-values, 4.0 and 4.3, respectively. Of the five partners contributing with full data sets, 80% could obtain the same rank order for the three model compounds using the SSPM (aprepitant > felodipine ≈ fenofibrate). The α-value is dependent on the experimental setup and can be used as a parameter to evaluate the uniformity of the data set. This study indicated that the SSPM was able to obtain the same rank order of the β-value between partners and, thus, that the SSPM may be used to classify compounds depending on their supersaturation propensity.
中文翻译:

小规模标准化过饱和和沉淀方法的实验室内和实验室间再现性研究
药物开发中大量难溶于水的化合物增加了对使制剂能够改善口服生物利用度的需求。一种常用的方法是在吸收位点(例如小肠)诱导过饱和,从而增加可用于吸收的溶解化合物的量。但是,由于成核的随机性,过饱和的药物递送系统可能导致人际和人际变异。相对于过饱和水平定义可行范围的能力是成功配制的关键因素。因此,需要一种体外方法,从中可以以可再现的方式定义化合物过饱和的能力。因此,本研究调查了体外的重现性小规模标准化过饱和和沉淀法(SSPM)。首先进行了非洛地平的实验室内可重复性研究,然后由七个合作伙伴提供了三种模型化合物的数据。aprepitant,非洛地平和非诺贝特,以确定SSPM的实验室间可重复性。SSPM的第一部分确定了每种化合物的表观过饱和度(aDS)。每个合作伙伴独立确定最大可能aDS,并在SSPM中诱发其确定的最大可能aDS的100%,87.5%,75%和50%。为了确定诱导时间(t ind),获得了过饱和和随后的沉淀的浓度-时间曲线。)用于可检测到的降水。数据表明伴侣之间t ind和aDS的绝对值不直接可比,但是,在数据线性化后,基于β值获得了三种模型化合物的可再现等级顺序,其定义为斜率ln(t ind)与ln(aDS)-2的关系阴谋。该图的线性回归表明,阿瑞匹坦的β值最高,为15.1,而非洛地平和非诺贝特的β值相当,分别为4.0和4.3。在提供完整数据集的五个合作伙伴中,有80%可以使用SSPM(阿瑞匹坦>非洛地平≈非诺贝特)获得三种模型化合物的相同等级顺序。α值取决于实验设置,可以用作评估数据集均匀性的参数。这项研究表明,SSPM能够在伙伴之间获得相同的β值排名,因此,SSPM可以根据化合物的过饱和度来对其进行分类。
更新日期:2017-11-08
中文翻译:

小规模标准化过饱和和沉淀方法的实验室内和实验室间再现性研究
药物开发中大量难溶于水的化合物增加了对使制剂能够改善口服生物利用度的需求。一种常用的方法是在吸收位点(例如小肠)诱导过饱和,从而增加可用于吸收的溶解化合物的量。但是,由于成核的随机性,过饱和的药物递送系统可能导致人际和人际变异。相对于过饱和水平定义可行范围的能力是成功配制的关键因素。因此,需要一种体外方法,从中可以以可再现的方式定义化合物过饱和的能力。因此,本研究调查了体外的重现性小规模标准化过饱和和沉淀法(SSPM)。首先进行了非洛地平的实验室内可重复性研究,然后由七个合作伙伴提供了三种模型化合物的数据。aprepitant,非洛地平和非诺贝特,以确定SSPM的实验室间可重复性。SSPM的第一部分确定了每种化合物的表观过饱和度(aDS)。每个合作伙伴独立确定最大可能aDS,并在SSPM中诱发其确定的最大可能aDS的100%,87.5%,75%和50%。为了确定诱导时间(t ind),获得了过饱和和随后的沉淀的浓度-时间曲线。)用于可检测到的降水。数据表明伴侣之间t ind和aDS的绝对值不直接可比,但是,在数据线性化后,基于β值获得了三种模型化合物的可再现等级顺序,其定义为斜率ln(t ind)与ln(aDS)-2的关系阴谋。该图的线性回归表明,阿瑞匹坦的β值最高,为15.1,而非洛地平和非诺贝特的β值相当,分别为4.0和4.3。在提供完整数据集的五个合作伙伴中,有80%可以使用SSPM(阿瑞匹坦>非洛地平≈非诺贝特)获得三种模型化合物的相同等级顺序。α值取决于实验设置,可以用作评估数据集均匀性的参数。这项研究表明,SSPM能够在伙伴之间获得相同的β值排名,因此,SSPM可以根据化合物的过饱和度来对其进行分类。



























 京公网安备 11010802027423号
京公网安备 11010802027423号