当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbene-Catalyzed Formal [5 + 5] Reaction for Coumarin Construction and Total Synthesis of Defucogilvocarcins
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03102 Xuan Huang 1 , Tingshun Zhu 1 , Zhijian Huang 1 , Yuexia Zhang 1 , Zhichao Jin 1, 2 , Giuseppe Zanoni 2 , Yonggui Robin Chi 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03102 Xuan Huang 1 , Tingshun Zhu 1 , Zhijian Huang 1 , Yuexia Zhang 1 , Zhichao Jin 1, 2 , Giuseppe Zanoni 2 , Yonggui Robin Chi 1
Affiliation
|
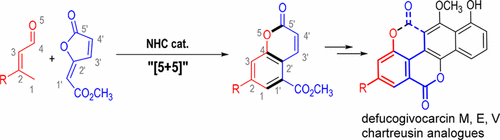
An N-heterocyclic carbene-catalyzed formal [5 + 5] reaction between enals and furanones that generates multisubstituted coumarins in a single step is reported. Five atoms in each of the substrates are involved in this catalytic process to form a benzene and a lactone ring. The power of the method is further demonstrated in metal-free total syntheses of several natural products (defucogilvocarcins M, E, and V) bearing coumarin as the key structural motif.
中文翻译:

碳催化的香豆素构建和全合成脱呋基新霉素的形式[5 + 5]反应
据报道,在烯醛和呋喃酮之间的N杂环卡宾催化的正式[5 + 5]反应可在一个步骤中生成多取代的香豆素。每个底物中的五个原子参与该催化过程以形成苯和内酯环。该方法的功效在以香豆素为关键结构基序的几种天然产物(去甲福尔霉素M,E和V)的无金属全合成中得到了进一步证明。
更新日期:2017-11-07
中文翻译:

碳催化的香豆素构建和全合成脱呋基新霉素的形式[5 + 5]反应
据报道,在烯醛和呋喃酮之间的N杂环卡宾催化的正式[5 + 5]反应可在一个步骤中生成多取代的香豆素。每个底物中的五个原子参与该催化过程以形成苯和内酯环。该方法的功效在以香豆素为关键结构基序的几种天然产物(去甲福尔霉素M,E和V)的无金属全合成中得到了进一步证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号