当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Highly Regioselective Azide–Ynamide Cycloaddition to Access 5-Amido Fully Substituted 1,2,3-Triazoles under Mild, Air, Aqueous, and Bioorthogonal Conditions
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03123 Wangze Song 1, 2 , Nan Zheng 2, 3
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03123 Wangze Song 1, 2 , Nan Zheng 2, 3
Affiliation
|
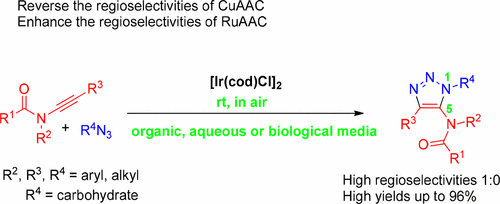
A highly regioselective method to access 5-amido fully substituted 1,2,3-triazoles by iridium-catalyzed azide–ynamide cycloaddition under mild, air, aqueous, and bioorthogonal conditions is reported. The excellent regioselectivities may derive from the strong coordination between the carbonyl oxygen of ynamide and the π-acidic iridium. Since the iridium ion is insensitive to oxygen/water and exhibits low cytotoxicity, it could catalyze this reaction in both organic and biological environments efficiently. Preparation in gram-scale and application in carbohydrates highlight this method.
中文翻译:

在温和,空气,水和生物正交条件下,铱催化的高区域选择性叠氮化物-亚酰胺环加成反应可得到5-氨基完全取代的1,2,3-三唑
据报道,在温和的,空气,水和生物正交条件下,通过铱催化的叠氮化物-酰胺基环加成反应可得到5-氨基完全取代的1,2,3-三唑的高度区域选择性方法。优异的区域选择性可以源自于酰胺的羰基氧与π-酸性铱之间的强配位。由于铱离子对氧气/水不敏感并且显示出低的细胞毒性,因此它可以在有机和生物环境中有效地催化该反应。以克为单位的制备和在碳水化合物中的应用突出了该方法。
更新日期:2017-11-07
中文翻译:

在温和,空气,水和生物正交条件下,铱催化的高区域选择性叠氮化物-亚酰胺环加成反应可得到5-氨基完全取代的1,2,3-三唑
据报道,在温和的,空气,水和生物正交条件下,通过铱催化的叠氮化物-酰胺基环加成反应可得到5-氨基完全取代的1,2,3-三唑的高度区域选择性方法。优异的区域选择性可以源自于酰胺的羰基氧与π-酸性铱之间的强配位。由于铱离子对氧气/水不敏感并且显示出低的细胞毒性,因此它可以在有机和生物环境中有效地催化该反应。以克为单位的制备和在碳水化合物中的应用突出了该方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号