当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
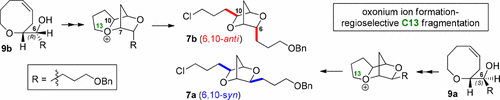
Construction of 6,10-syn- and -anti-2,5-Dioxabicyclo[2.2.1]heptane Skeletons via Oxonium Ion Formation/Fragmentation: Prediction of Structure of (E)-Ocellenyne by NMR Calculation
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03226 Daeyeon Jeong 1 , Te-ik Sohn 1 , Jong Yup Kim 1 , Gyudong Kim 1 , Deukjoon Kim 1 , Robert S. Paton 2
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03226 Daeyeon Jeong 1 , Te-ik Sohn 1 , Jong Yup Kim 1 , Gyudong Kim 1 , Deukjoon Kim 1 , Robert S. Paton 2
Affiliation
|
A highly efficient and stereoselective route to potential synthetic intermediates for ocellenyne and related C15 acetogenin natural products with 6,10-syn- and 6,10-anti-2,5-dioxabicyclo[2.2.1]heptane core structures has been developed by means of an iterative biogenesis-inspired oxonium ion formation/fragmentation sequence. In accordance with chemical transformations, the most likely stereostructure for (E)-ocellenyne on the basis of GIAO 13C NMR calculations possesses a 6,10-anti-2,5-dioxabicyclo[2.2.1]heptane core, as predicted from a plausible biosynthetic pathway, instead of the spectroscopically proposed 6,10-syn-2,5-dioxabicyclo[2.2.1]heptane skeleton.
中文翻译:

氧离子形成/断裂法构建6,10-顺式和反式-2,5-二氧杂双环[2.2.1]庚烷骨架:(E)-Ocellenyne结构的NMR预测
通过开发一种高效,立体选择性的途径,可开发出具有6,10-顺-和6,10-抗-2,5-二氧杂双环[2.2.1]庚烷核心结构的奥氏炔和相关的C 15产乙酸原天然产物的潜在合成中间体。迭代的生物发生启发式的氧离子形成/片段化序列的方法。根据化学转化,根据GIAO 13 C NMR计算,(E)-邻二炔的最可能的立体结构具有6,10-抗-2,5-二氧杂双环[2.2.1]庚烷核,从合理的生物合成途径,而不是光谱学上提出的6,10- syn-2,5-二氧杂双环[2.2.1]庚烷骨架。
更新日期:2017-11-07
中文翻译:

氧离子形成/断裂法构建6,10-顺式和反式-2,5-二氧杂双环[2.2.1]庚烷骨架:(E)-Ocellenyne结构的NMR预测
通过开发一种高效,立体选择性的途径,可开发出具有6,10-顺-和6,10-抗-2,5-二氧杂双环[2.2.1]庚烷核心结构的奥氏炔和相关的C 15产乙酸原天然产物的潜在合成中间体。迭代的生物发生启发式的氧离子形成/片段化序列的方法。根据化学转化,根据GIAO 13 C NMR计算,(E)-邻二炔的最可能的立体结构具有6,10-抗-2,5-二氧杂双环[2.2.1]庚烷核,从合理的生物合成途径,而不是光谱学上提出的6,10- syn-2,5-二氧杂双环[2.2.1]庚烷骨架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号