当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influences of the heme-lysine crosslink in cytochrome P460 over redox catalysis and nitric oxide sensitivity†
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7sc03450d Avery C. Vilbert 1, 2, 3, 4, 5 , Jonathan D. Caranto 1, 2, 3, 4, 5 , Kyle M. Lancaster 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7sc03450d Avery C. Vilbert 1, 2, 3, 4, 5 , Jonathan D. Caranto 1, 2, 3, 4, 5 , Kyle M. Lancaster 1, 2, 3, 4, 5
Affiliation
|
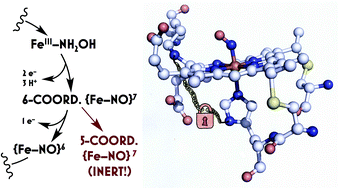
Ammonia (NH3)-oxidizing bacteria (AOB) derive total energy for life from the multi-electron oxidation of NH3 to nitrite (NO2−). One obligate intermediate of this metabolism is hydroxylamine (NH2OH), which can be oxidized to the potent greenhouse agent nitrous oxide (N2O) by the AOB enzyme cytochrome (cyt) P460. We have now spectroscopically characterized a 6-coordinate (6c) {FeNO}7 intermediate on the NH2OH oxidation pathway of cyt P460. This species has two fates: it can either be oxidized to the {FeNO}6 that then undergoes attack by NH2OH to ultimately generate N2O, or it can lose its axial His ligand, thus generating a stable, off-pathway 5-coordinate (5c) {FeNO}7 species. We show that the wild type (WT) cyt P460 exhibits a slow nitric oxide (NO)-independent conversion (kHis-off = 2.90 × 10−3 s−1), whereas a cross-link-deficient Lys70Tyr cyt P460 mutant protein underwent His dissociation via both a NO-independent (kHis-off = 3.8 × 10−4 s−1) and a NO-dependent pathway [kHis-off(NO) = 790 M−1 s−1]. Eyring analyses of the NO-independent pathways for these two proteins revealed a significantly larger (ca. 27 cal mol−1 K−1) activation entropy (ΔS‡) in the cross-link-deficient mutant. Our results suggest that the Lys–heme cross-link confers rigidity to the positioning of the heme P460 cofactor to avoid the fast NO-dependent His dissociation pathway and subsequent formation of the off-pathway 5c {FeNO}7 species. The relevance of these findings to NO signaling proteins such as heme-nitric oxide/oxygen binding (H-NOX) is also discussed.
中文翻译:

细胞色素P460中的血红素-赖氨酸交联对氧化还原催化和一氧化氮敏感性的影响†
氨(NH 3)氧化菌(AOB)导出用于生命总能量从NH的多电子氧化3为亚硝酸盐(NO 2 - )。这种代谢的一种专性中间体是羟胺(NH 2 OH),它可以被AOB酶细胞色素(cyt)P460氧化成有效的温室效应一氧化二氮(N 2 O)。现在,我们已经在细胞色素P460的NH 2 OH氧化途径上以光谱形式表征了6坐标(6c){FeNO} 7中间体。这个物种有两个命运:它可以被氧化成{FeNO} 6,然后受到NH 2 OH的攻击最终产生N 2。O,否则它可能会失去其轴向His配体,从而生成稳定的,偏离路径的5坐标(5c){FeNO} 7种。我们显示野生型(WT)cyt P460表现出缓慢的一氧化氮(NO)独立转化(k His-off = 2.90×10 -3 s -1),而交联缺陷的Lys70Tyr cyt P460突变蛋白接受他离解经由两者NO无关(ķ他断= 3.8×10 -4小号-1)和NO依赖性途径[ ķ他断(NO) = 790米之-1小号-1]。对这两种蛋白质的NO依赖性途径的Eyring分析表明,在交联缺陷型突变体中,活化熵(ΔS ‡)明显更大(约27 cal mol -1 K -1)。我们的结果表明,Lys-血红素交联赋予血红素P460辅因子以刚性,以避免快速的NO依赖性His分解途径和随后形成的非通路5c {FeNO} 7物种。还讨论了这些发现与NO信号蛋白(如一氧化二氮/氧结合(H-NOX))的相关性。
更新日期:2017-11-07
中文翻译:

细胞色素P460中的血红素-赖氨酸交联对氧化还原催化和一氧化氮敏感性的影响†
氨(NH 3)氧化菌(AOB)导出用于生命总能量从NH的多电子氧化3为亚硝酸盐(NO 2 - )。这种代谢的一种专性中间体是羟胺(NH 2 OH),它可以被AOB酶细胞色素(cyt)P460氧化成有效的温室效应一氧化二氮(N 2 O)。现在,我们已经在细胞色素P460的NH 2 OH氧化途径上以光谱形式表征了6坐标(6c){FeNO} 7中间体。这个物种有两个命运:它可以被氧化成{FeNO} 6,然后受到NH 2 OH的攻击最终产生N 2。O,否则它可能会失去其轴向His配体,从而生成稳定的,偏离路径的5坐标(5c){FeNO} 7种。我们显示野生型(WT)cyt P460表现出缓慢的一氧化氮(NO)独立转化(k His-off = 2.90×10 -3 s -1),而交联缺陷的Lys70Tyr cyt P460突变蛋白接受他离解经由两者NO无关(ķ他断= 3.8×10 -4小号-1)和NO依赖性途径[ ķ他断(NO) = 790米之-1小号-1]。对这两种蛋白质的NO依赖性途径的Eyring分析表明,在交联缺陷型突变体中,活化熵(ΔS ‡)明显更大(约27 cal mol -1 K -1)。我们的结果表明,Lys-血红素交联赋予血红素P460辅因子以刚性,以避免快速的NO依赖性His分解途径和随后形成的非通路5c {FeNO} 7物种。还讨论了这些发现与NO信号蛋白(如一氧化二氮/氧结合(H-NOX))的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号