当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microenvironmental Stiffness of 3D Polymeric Structures to Study Invasive Rates of Cancer Cells
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-11-06 , DOI: 10.1002/adhm.201700888 Enrico Domenico Lemma 1, 2 , Barbara Spagnolo 1 , Francesco Rizzi 1 , Stefania Corvaglia 1 , Marco Pisanello 1, 2 , Massimo De Vittorio 1, 2 , Ferruccio Pisanello 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-11-06 , DOI: 10.1002/adhm.201700888 Enrico Domenico Lemma 1, 2 , Barbara Spagnolo 1 , Francesco Rizzi 1 , Stefania Corvaglia 1 , Marco Pisanello 1, 2 , Massimo De Vittorio 1, 2 , Ferruccio Pisanello 1
Affiliation
|
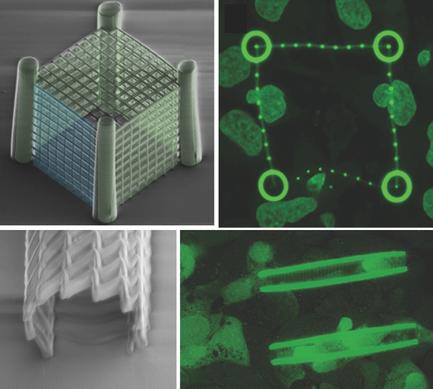
Cells are highly dynamic elements, continuously interacting with the extracellular environment. Mechanical forces sensed and applied by cells are responsible for cellular adhesion, motility, and deformation, and are heavily involved in determining cancer spreading and metastasis formation. Cell/extracellular matrix interactions are commonly analyzed with the use of hydrogels and 3D microfabricated scaffolds. However, currently available techniques have a limited control over the stiffness of microscaffolds and do not allow for separating environmental properties from biological processes in driving cell mechanical behavior, including nuclear deformability and cell invasiveness. Herein, a new approach is presented to study tumor cell invasiveness by exploiting an innovative class of polymeric scaffolds based on two‐photon lithography to control the stiffness of deterministic microenvironments in 3D. This is obtained by fine‐tuning of the laser power during the lithography, thus locally modifying both structural and mechanical properties in the same fabrication process. Cage‐like structures and cylindric stent‐like microscaffolds are fabricated with different Young's modulus and stiffness gradients, allowing obtaining new insights on the mechanical interplay between tumor cells and the surrounding environments. In particular, cell invasion is mostly driven by softer architectures, and the introduction of 3D stiffness “weak spots” is shown to boost the rate at which cancer cells invade the scaffolds. The possibility to modulate structural compliance also allowed estimating the force distribution exerted by a single cell on the scaffold, revealing that both pushing and pulling forces are involved in the cell–structure interaction. Overall, exploiting this method to obtain a wide range of 3D architectures with locally engineered stiffness can pave the way for unique applications to study tumor cell dynamics.
中文翻译:

3D高分子结构的微环境刚度研究癌细胞的侵袭率
细胞是高度动态的元素,与细胞外环境不断相互作用。细胞感测和施加的机械力负责细胞粘附,运动和变形,并大量参与确定癌症的扩散和转移形成。通常使用水凝胶和3D微型支架来分析细胞/细胞外基质的相互作用。然而,当前可用的技术对微支架的刚度具有有限的控制,并且不允许在驱动细胞机械行为(包括核可变形性和细胞侵袭性)的过程中将环境特性与生物过程分开。在此处,通过利用基于双光子光刻技术的创新类聚合物支架来控制3D确定性微环境的刚度,提出了一种研究肿瘤细胞侵袭性的新方法。这是通过在光刻过程中微调激光功率而获得的,从而在同一制造过程中局部修改了结构和机械性能。笼状结构和圆柱形支架式微支架的制造具有不同的杨氏模量和刚度梯度,从而获得了关于肿瘤细胞与周围环境之间机械相互作用的新见解。特别是,细胞入侵主要是由较软的体系结构驱动的,并且3D刚度“弱点”的引入显示出可以提高癌细胞入侵支架的速度。调节结构顺应性的可能性还允许估计单个细胞在支架上施加的力分布,这表明推力和拉力都参与了细胞-结构相互作用。总体而言,利用此方法获得具有局部工程刚度的各种3D架构可以为研究肿瘤细胞动力学的独特应用铺平道路。
更新日期:2017-11-06
中文翻译:

3D高分子结构的微环境刚度研究癌细胞的侵袭率
细胞是高度动态的元素,与细胞外环境不断相互作用。细胞感测和施加的机械力负责细胞粘附,运动和变形,并大量参与确定癌症的扩散和转移形成。通常使用水凝胶和3D微型支架来分析细胞/细胞外基质的相互作用。然而,当前可用的技术对微支架的刚度具有有限的控制,并且不允许在驱动细胞机械行为(包括核可变形性和细胞侵袭性)的过程中将环境特性与生物过程分开。在此处,通过利用基于双光子光刻技术的创新类聚合物支架来控制3D确定性微环境的刚度,提出了一种研究肿瘤细胞侵袭性的新方法。这是通过在光刻过程中微调激光功率而获得的,从而在同一制造过程中局部修改了结构和机械性能。笼状结构和圆柱形支架式微支架的制造具有不同的杨氏模量和刚度梯度,从而获得了关于肿瘤细胞与周围环境之间机械相互作用的新见解。特别是,细胞入侵主要是由较软的体系结构驱动的,并且3D刚度“弱点”的引入显示出可以提高癌细胞入侵支架的速度。调节结构顺应性的可能性还允许估计单个细胞在支架上施加的力分布,这表明推力和拉力都参与了细胞-结构相互作用。总体而言,利用此方法获得具有局部工程刚度的各种3D架构可以为研究肿瘤细胞动力学的独特应用铺平道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号