当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Pharmacology, and Molecular Docking Studies on 6-Desoxo-N-methylmorphinans as Potent μ-Opioid Receptor Agonists
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01363 Maria Dumitrascuta 1 , Tanila Ben Haddou 1 , Elena Guerrieri 1 , Stefan M. Noha 1 , Lea Schläfer 1 , Helmut Schmidhammer 1 , Mariana Spetea 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01363 Maria Dumitrascuta 1 , Tanila Ben Haddou 1 , Elena Guerrieri 1 , Stefan M. Noha 1 , Lea Schläfer 1 , Helmut Schmidhammer 1 , Mariana Spetea 1
Affiliation
|
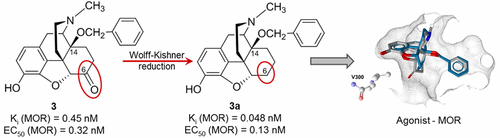
Position 6 of the morphinan skeleton plays a key role in the μ-opioid receptor (MOR) activity in vitro and in vivo. We describe the consequence of the 6-carbonyl group deletion in N-methylmorphinan-6-ones 1–4 on ligand–MOR interaction, signaling, and antinociception. While 6-desoxo compounds 1a, 2a, and 4a show similar profiles to their 6-keto counterparts, the 6-desoxo-14-benzyloxy substituted 3a displays significantly increased MOR binding and agonist potency and a distinct binding mode compared with its analogue 3.
中文翻译:

6-Desoxo- N-甲基吗啡喃作为有效的μ阿片受体激动剂的合成,药理学和分子对接研究
吗啡喃骨架的6位在体外和体内的μ阿片受体(MOR)活性中起着关键作用。我们描述了在6 -羰基缺失的结果Ñ -methylmorphinan -6-酮1 - 4上的配体- MOR相互作用,信号,和抗伤害感受作用。虽然6-desoxo化合物1a,2a和4a与它们的6-keto对应物具有相似的分布,但6-desoxo-14-苄氧基取代的3a与其类似物3相比,其MOR结合力和激动剂效能显着提高,结合方式也不同。
更新日期:2017-11-05
中文翻译:

6-Desoxo- N-甲基吗啡喃作为有效的μ阿片受体激动剂的合成,药理学和分子对接研究
吗啡喃骨架的6位在体外和体内的μ阿片受体(MOR)活性中起着关键作用。我们描述了在6 -羰基缺失的结果Ñ -methylmorphinan -6-酮1 - 4上的配体- MOR相互作用,信号,和抗伤害感受作用。虽然6-desoxo化合物1a,2a和4a与它们的6-keto对应物具有相似的分布,但6-desoxo-14-苄氧基取代的3a与其类似物3相比,其MOR结合力和激动剂效能显着提高,结合方式也不同。



























 京公网安备 11010802027423号
京公网安备 11010802027423号