当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Lewis superacid Al[N(C6F5)2]3 and its higher homolog Ga[N(C6F5)2]3 – structural features, theoretical investigation and reactions of a metal amide with higher fluoride ion affinity than SbF5
Chemical Science ( IF 8.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7sc03988c J. F. Kögel 1, 2, 3 , D. A. Sorokin 1, 2, 3 , A. Khvorost 1, 2, 3 , M. Scott 1, 2, 3 , K. Harms 1, 2, 3 , D. Himmel 4, 5, 6, 7, 8 , I. Krossing 4, 5, 6, 7, 8 , J. Sundermeyer 1, 2, 3
Chemical Science ( IF 8.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7sc03988c J. F. Kögel 1, 2, 3 , D. A. Sorokin 1, 2, 3 , A. Khvorost 1, 2, 3 , M. Scott 1, 2, 3 , K. Harms 1, 2, 3 , D. Himmel 4, 5, 6, 7, 8 , I. Krossing 4, 5, 6, 7, 8 , J. Sundermeyer 1, 2, 3
Affiliation
|
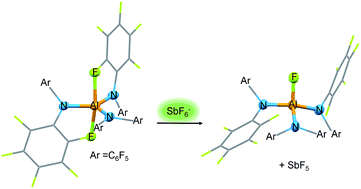
Herein we present the synthesis of the two Lewis acids Al[N(C6F5)2]3 (ALTA) and Ga[N(C6F5)2]3 (GATA) via salt elimination reactions. The metal complexes were characterized by NMR-spectroscopic methods and X-ray diffraction analysis revealing the stabilization of the highly Lewis acidic metal centers by secondary metal–fluorine contacts. The Lewis acidic properties of Al[N(C6F5)2]3 and Ga[N(C6F5)2]3 are demonstrated by reactions with Lewis bases resulting in the formation of metallates accompanied by crucial structural changes. The two metallates [Cs(Tol)3]+[FAl(N(C6F5)2)3]− and [AsPh4]+[ClGa(N(C6F5)2)3]− contain interesting weakly coordinating anions. The reaction of Al[N(C6F5)2]3 with trityl fluoride yielded [CPh3]+[FAl(N(C6F5)2)3]− which could find application in the activation of metallocene polymerization catalysts. The qualitative Lewis acidity of Al[N(C6F5)2]3 and Ga[N(C6F5)2]3 was investigated by means of competition experiments for chloride ions in solution. DFT calculations yielded fluoride ion affinities in the gas phase (FIA) of 555 kJ mol−1 for Al[N(C6F5)2]3 and 472 kJ mol−1 for Ga[N(C6F5)2]3. Thus, Al[N(C6F5)2]3 can be considered a Lewis superacid with a fluoride affinity higher than SbF5 (493 kJ mol−1) whereas the FIA of the corresponding gallium complex is slightly below the threshold to Lewis superacidity.
中文翻译:

刘易斯超酸Al [N(C 6 F 5)2 ] 3及其较高的同系物Ga [N(C 6 F 5)2 ] 3-氟化物离子亲和力比SbF高的金属酰胺的结构特征,理论研究和反应5
在这里,我们介绍了通过盐消除反应合成两种路易斯酸Al [N(C 6 F 5)2 ] 3(ALTA)和Ga [N(C 6 F 5)2 ] 3(GATA)的方法。通过NMR光谱法和X射线衍射分析对金属配合物进行了表征,揭示了次级金属与氟的接触稳定了高路易斯酸性金属中心。Al [N(C 6 F 5)2 ] 3和Ga [N(C 6 F 5)2 ] 3的路易斯酸性通过与路易斯碱的反应证明了这一点,该反应导致形成金属盐并伴有关键的结构变化。两种金属盐[Cs(Tol)3 ] + [FAl(N(C 6 F 5)2)3 ] -和[AsPh 4 ] + [ClGa(N(C 6 F 5)2)3 ] -含有趣的弱配位阴离子。Al [N(C 6 F 5)2 ] 3与三苯甲基氟的反应生成[CPh 3 ] +[FAl(N(C 6 F 5)2)3 ] -可用于茂金属聚合催化剂的活化。通过溶液中氯离子的竞争实验研究了Al [N(C 6 F 5)2 ] 3和Ga [N(C 6 F 5)2 ] 3的定性路易斯酸度。DFT计算得出,对于Al [N(C 6 F 5)2 ] 3和472 kJ mol ,气相(FIA)的氟离子亲合力为555 kJ mol -1对于Ga [N(C 6 F 5) 2 ] 3,为-1。因此,可以认为Al [N(C 6 F 5) 2 ] 3具有较高的氟化物亲和力,其路易斯亲合力高于SbF 5(493 kJ mol -1),而相应的镓络合物的FIA略低于路易斯的阈值。超酸度。
更新日期:2017-11-03
中文翻译:

刘易斯超酸Al [N(C 6 F 5)2 ] 3及其较高的同系物Ga [N(C 6 F 5)2 ] 3-氟化物离子亲和力比SbF高的金属酰胺的结构特征,理论研究和反应5
在这里,我们介绍了通过盐消除反应合成两种路易斯酸Al [N(C 6 F 5)2 ] 3(ALTA)和Ga [N(C 6 F 5)2 ] 3(GATA)的方法。通过NMR光谱法和X射线衍射分析对金属配合物进行了表征,揭示了次级金属与氟的接触稳定了高路易斯酸性金属中心。Al [N(C 6 F 5)2 ] 3和Ga [N(C 6 F 5)2 ] 3的路易斯酸性通过与路易斯碱的反应证明了这一点,该反应导致形成金属盐并伴有关键的结构变化。两种金属盐[Cs(Tol)3 ] + [FAl(N(C 6 F 5)2)3 ] -和[AsPh 4 ] + [ClGa(N(C 6 F 5)2)3 ] -含有趣的弱配位阴离子。Al [N(C 6 F 5)2 ] 3与三苯甲基氟的反应生成[CPh 3 ] +[FAl(N(C 6 F 5)2)3 ] -可用于茂金属聚合催化剂的活化。通过溶液中氯离子的竞争实验研究了Al [N(C 6 F 5)2 ] 3和Ga [N(C 6 F 5)2 ] 3的定性路易斯酸度。DFT计算得出,对于Al [N(C 6 F 5)2 ] 3和472 kJ mol ,气相(FIA)的氟离子亲合力为555 kJ mol -1对于Ga [N(C 6 F 5) 2 ] 3,为-1。因此,可以认为Al [N(C 6 F 5) 2 ] 3具有较高的氟化物亲和力,其路易斯亲合力高于SbF 5(493 kJ mol -1),而相应的镓络合物的FIA略低于路易斯的阈值。超酸度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号