当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
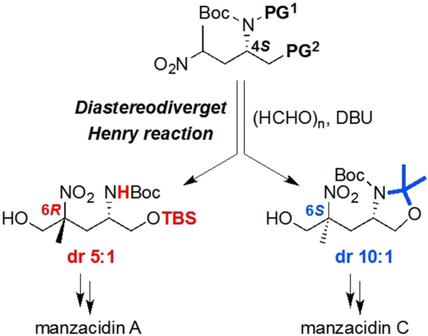
Diastereodivergent Henry Reaction for the Stereoselective Construction of Nitrogen-Containing Tetrasubstituted Carbons: Application to Total Synthesis of Manzacidins A and C
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-03 06:52:11 , DOI: 10.1002/ajoc.201700568 Takayuki Kudoh 1 , Yuya Araki 1 , Natsumi Miyoshi 1 , Mizuho Tanioka 1 , Akira Sakakura 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-03 06:52:11 , DOI: 10.1002/ajoc.201700568 Takayuki Kudoh 1 , Yuya Araki 1 , Natsumi Miyoshi 1 , Mizuho Tanioka 1 , Akira Sakakura 1
Affiliation
|
Henry reaction of chiral secondary nitroalkanes constructed nitrogen-containing tetrasubstited carbon stereocenters with high diastereoselectivity. Protecting groups of the nitroalkanes played a key role to control the diastereoselectivity. Using the Henry adducts as key intermediates, a total synthesis of manzacidins A and C has been achieved.
中文翻译:

含氮四取代碳的立体选择性构型的非对映发散亨利反应:在Manzacidins A和C的全合成中的应用
手性仲硝基烷烃的亨利反应以高非对映选择性构建了含氮的四取代碳立体中心。硝基烷的保护基在控制非对映选择性中起关键作用。使用亨利加合物作为关键中间体,已实现了全合成的山梨酸苷A和C。
更新日期:2017-11-03
中文翻译:

含氮四取代碳的立体选择性构型的非对映发散亨利反应:在Manzacidins A和C的全合成中的应用
手性仲硝基烷烃的亨利反应以高非对映选择性构建了含氮的四取代碳立体中心。硝基烷的保护基在控制非对映选择性中起关键作用。使用亨利加合物作为关键中间体,已实现了全合成的山梨酸苷A和C。


























 京公网安备 11010802027423号
京公网安备 11010802027423号