当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship Studies of Pyrimido[5,4-b]indoles as Selective Toll-Like Receptor 4 Ligands
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00797 Michael Chan 1 , Yuhei Kakitsubata 2 , Tomoko Hayashi 1 , Alast Ahmadi 1 , Shiyin Yao 1 , Nikunj M. Shukla 1 , Shin-ya Oyama 2 , Akihito Baba 2 , Brandon Nguyen 1 , Maripat Corr 3 , Yasuo Suda 2 , Dennis A. Carson 1, 3 , Howard B. Cottam 1, 3 , Masahiro Wakao 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00797 Michael Chan 1 , Yuhei Kakitsubata 2 , Tomoko Hayashi 1 , Alast Ahmadi 1 , Shiyin Yao 1 , Nikunj M. Shukla 1 , Shin-ya Oyama 2 , Akihito Baba 2 , Brandon Nguyen 1 , Maripat Corr 3 , Yasuo Suda 2 , Dennis A. Carson 1, 3 , Howard B. Cottam 1, 3 , Masahiro Wakao 1, 2
Affiliation
|
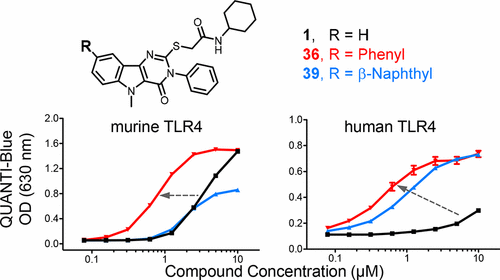
Previous high throughput screening studies led to the discovery of two novel, nonlipid-like chemotypes as Toll-like receptor 4 (TLR4) agonists. One of these chemotypes, the pyrimido[5,4-b]indoles, was explored for structure–activity relationship trends relative to production of TLR4 dependent cytokines/chemokines, resulting in a semioptimized lead (compound 1) that provided a starting point for further optimization studies. In this report, compounds belonging to three areas of structural modification were evaluated for biological activity using murine and human TLR4 reporter cells, primary murine bone marrow derived dendritic cells, and human peripheral blood mononuclear cells. The compounds bearing certain aryl groups at the C8 position, such as phenyl (36) and β-naphthyl (39), had potencies significantly greater than compound 1. Compound 36 displayed human TLR4 agonist activity at submicromolar concentrations. The computational analysis suggests that the improved potency of these C8-aryl derivatives may be the result of additional binding interactions at the interface of the TLR4/myeloid differentiation protein-2 (MD-2) complex.
中文翻译:

嘧啶并[5,4- b ]吲哚作为选择性收费受体4配体的构效关系研究
先前的高通量筛选研究导致发现了两种新型的非脂质样化学型,作为Toll样受体4(TLR4)激动剂。探索了这些化学型之一,嘧啶并[5,4- b ]吲哚相对于TLR4依赖性细胞因子/趋化因子的产生的结构-活性关系趋势,从而产生了半优化的铅(化合物1),为进一步研究提供了起点优化研究。在该报告中,使用鼠类和人类TLR4报告基因细胞,原代小鼠骨髓来源的树突状细胞和人外周血单核细胞对属于三个结构修饰区域的化合物的生物活性进行了评估。在C8位置带有某些芳基的化合物,例如苯基(36)和β-萘基(39)的效力显着大于化合物1。化合物36在亚微摩尔浓度下显示人TLR4激动剂活性。计算分析表明,这些C8-芳基衍生物的效力提高可能是由于TLR4 /髓样分化蛋白2(MD-2)复合物界面上其他结合相互作用的结果。
更新日期:2017-11-03
中文翻译:

嘧啶并[5,4- b ]吲哚作为选择性收费受体4配体的构效关系研究
先前的高通量筛选研究导致发现了两种新型的非脂质样化学型,作为Toll样受体4(TLR4)激动剂。探索了这些化学型之一,嘧啶并[5,4- b ]吲哚相对于TLR4依赖性细胞因子/趋化因子的产生的结构-活性关系趋势,从而产生了半优化的铅(化合物1),为进一步研究提供了起点优化研究。在该报告中,使用鼠类和人类TLR4报告基因细胞,原代小鼠骨髓来源的树突状细胞和人外周血单核细胞对属于三个结构修饰区域的化合物的生物活性进行了评估。在C8位置带有某些芳基的化合物,例如苯基(36)和β-萘基(39)的效力显着大于化合物1。化合物36在亚微摩尔浓度下显示人TLR4激动剂活性。计算分析表明,这些C8-芳基衍生物的效力提高可能是由于TLR4 /髓样分化蛋白2(MD-2)复合物界面上其他结合相互作用的结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号