European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.ejmech.2017.10.083 Beatriz Balsera , José Mulet , Salvador Sala , Francisco Sala , Roberto de la Torre-Martínez , Sara González-Rodríguez , Adrián Plata , Lieve Naesens , Asia Fernández-Carvajal , Antonio Ferrer-Montiel , Manuel Criado , María Jesús Pérez de Vega , Rosario González-Muñiz
|
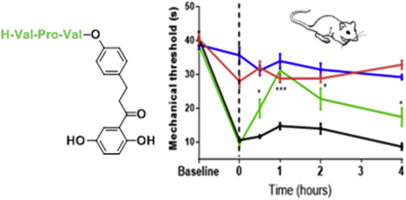
α7 Nicotinic acetylcholine receptors (nAChRs) are ion channels implicated in a number of CNS pathological processes, including pain and psychiatric, cognitive and inflammatory diseases. Comparing with orthosteric agonism, positive allosteric modulation of these channels constitutes an interesting approach to achieve selectivity versus other nicotinic receptors. We have recently described new chalcones and 1,3-diphenylpropanones as positive allosteric modulators (PAMs) of α7 nAChRs, which proved to have good analgesic activities but poor pharmacokinetic properties. Here we report the preparation of amino acid and peptide derivatives as prodrugs of these modulators with the aim of improving their in vivo biological activity. While the valine derivative showed very short half life in aqueous solutions to be considered a prodrug, Val-Val and Val-Pro-Val are suitable precursors of the parent 1,3-diphenylpropanones, via chemical and enzymatic transformation, respectively. Compounds 19 (Val-Val) and 21 (Val-Pro-Val), prodrugs of the 2′,5′,4-trihydroxy-1,3-diphenylpropan-1-one 3, showed significant antinociceptive activity in in vivo assays. The best compound, 21, displayed a better profile in the analgesia test than its parent compound 3, exhibiting about the same potency but long-lasting effects.
中文翻译:

具有镇痛活性的α7烟碱受体二苯基丙烷正构构调节剂的氨基酸和肽前药
α7烟碱乙酰胆碱受体(nAChRs)是离子通道,涉及许多CNS病理过程,包括疼痛和精神病,认知疾病和炎性疾病。与正构激动剂相比,这些通道的正构构调制相对于其他烟碱样受体构成了一种实现选择性的有趣方法。我们最近描述了新的查耳酮和1,3-二苯基丙烷作为α7nAChRs的正变构调节剂(PAM),它们被证明具有良好的镇痛活性,但药代动力学性能较差。在这里,我们报告了氨基酸和肽衍生物作为这些调节剂的前药的制备,目的是改善其体内活性生物活性。尽管缬氨酸衍生物在水溶液中显示出很短的半衰期,可以认为它们是前药,但Val-Val和Val-Pro-Val分别通过化学和酶促转化反应成为母体1,3-二苯基丙烷的合适前体。2',5',4-三羟基-1,3-二苯基丙烷-1-酮3的前药化合物19(Val-Val)和21(Val-Pro-Val)在体内试验中显示出显着的抗伤害感受活性。最好的化合物21在镇痛测试中显示出比其母体化合物3更好的特性,并表现出大致相同的功效,但效果持久。



























 京公网安备 11010802027423号
京公网安备 11010802027423号