European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.ejmech.2017.10.076 Ahmed T. Negmeldin , Joseph R. Knoff , Mary Kay H. Pflum
|
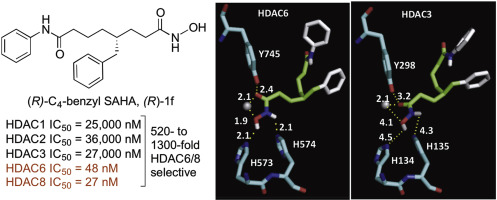
Histone deacetylase (HDAC) enzymes govern the post-translational acetylation state of lysine residues on protein substrates, leading to regulatory changes in cell function. Due to their role in cancers, HDAC proteins have emerged as promising targets for cancer treatment. Four HDAC inhibitors have been approved as anti-cancer therapeutics, including SAHA (Suberoylanilide hydroxamic acid, Vorinostat, Zolinza). SAHA is a nonselective HDAC inhibitor that targets most of the eleven HDAC isoforms. The nonselectivity of SAHA might account for its clinical side effects, but certainly limits its use as a chemical tool to study cancer-related HDAC cell biology. Herein, the nonselective HDAC inhibitor SAHA was modified at the C4 position of the linker to explore activity and selectivity. Several C4-modified SAHA analogs exhibited dual HDAC6/8 selectivity. Interestingly, (R)-C4-benzyl SAHA displayed 520- to 1300-fold selectivity for HDAC6 and HDAC8 over HDAC1, 2, and 3, with IC50 values of 48 and 27 nM with HDAC6 and 8, respectively. In cellulo testing of the inhibitors was consistent with the observed in vitro selectivity. Docking studies provided a structural rationale for selectivity. The C4-SAHA analogs represent useful chemical tools to understand the role of HDAC6 and HDAC8 in cancer biology and exciting lead compounds for targeting of both HDAC6 and HDAC8 in various cancers.
中文翻译:

组蛋白脱乙酰基酶抑制剂的结构要求:C4修饰的SAHA类似物具有双重HDAC6 / HDAC8选择性
组蛋白脱乙酰基酶(HDAC)酶控制蛋白质底物上赖氨酸残基的翻译后乙酰化状态,从而导致细胞功能的调节变化。由于其在癌症中的作用,HDAC蛋白已成为治疗癌症的有希望的靶标。四种HDAC抑制剂已被批准用作抗癌治疗剂,包括SAHA(Suberoylanilide异羟肟酸,Vorinostat,Zolinza)。SAHA是一种非选择性HDAC抑制剂,可靶向11种HDAC同工型中的大多数。SAHA的非选择性可能是其临床副作用的原因,但肯定会限制其作为研究癌症相关HDAC细胞生物学的化学工具的用途。在本文中,非选择性HDAC抑制剂SAHA在接头的C4位被修饰以探索活性和选择性。几种C4修饰的SAHA类似物表现出双重HDAC6 / 8选择性。R)-C4-苄基SAHA对HDAC6和HDAC8的选择性比HDAC1、2和3高520到1300倍,HDAC6和8的IC 50值分别为48和27 nM。在纤维素中测试抑制剂与观察到的体外选择性一致。对接研究为选择性提供了结构上的理由。C4-SAHA类似物代表有用的化学工具,可了解HDAC6和HDAC8在癌症生物学中的作用以及在多种癌症中靶向HDAC6和HDAC8的激动人心的先导化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号