当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Coordination Chemistry of N-2-Pyridylimidoyl-2-pyridylamidine: A Versatile Ligand with Multiple Coordination Sites
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.cgd.7b01232 Raúl Castañeda 1 , Andrew Hollingshead 1 , Bulat Gabidullin 1 , Jaclyn L. Brusso 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.cgd.7b01232 Raúl Castañeda 1 , Andrew Hollingshead 1 , Bulat Gabidullin 1 , Jaclyn L. Brusso 1
Affiliation
|
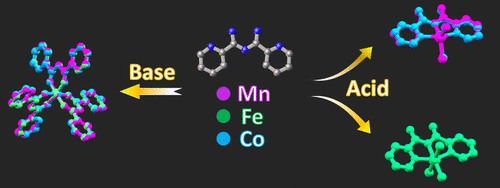
The research presented herein focuses on controlling the coordination environment of Mn, Fe, and Co in coordination complexes with the ligand N-2-pyridylimidoyl-2-pyridylamidine (Py2ImAm), which possesses both a bidentate and tridentate coordinating site. The synthesis and characterization of seven new metallic complexes are described, and these results indicate that the key factor dictating which coordination site of Py2ImAm the metal ion prefers is the presence (or absence) of a weak acid. More specifically, the presence of a weak acid directs the metal ion to the tridentate site of Py2ImAm, while neutral or basic conditions lead to coordination complexes in which the metal center is bound to the bidentate pocket.
中文翻译:

N -2-吡啶基亚甲基-2-吡啶基idine啶的配位化学的探索:具有多个配位位点的多功能配体。
本文提出的研究集中于控制与配体N -2-吡啶基酰亚胺基-2-吡啶基lam啶(Py 2 ImAm)配位的络合物中Mn,Fe和Co的配位环境,该配体同时具有二齿和三齿配位位点。描述了七个新的金属配合物的合成和表征,这些结果表明,决定金属离子偏爱Py 2 ImAm哪个配位点的关键因素是弱酸的存在(或不存在)。更具体地说,弱酸的存在将金属离子引导到Py 2 ImAm的三齿位点,而中性或基本条件会导致配位络合物,其中金属中心被束缚在双齿袋中。
更新日期:2017-10-31
中文翻译:

N -2-吡啶基亚甲基-2-吡啶基idine啶的配位化学的探索:具有多个配位位点的多功能配体。
本文提出的研究集中于控制与配体N -2-吡啶基酰亚胺基-2-吡啶基lam啶(Py 2 ImAm)配位的络合物中Mn,Fe和Co的配位环境,该配体同时具有二齿和三齿配位位点。描述了七个新的金属配合物的合成和表征,这些结果表明,决定金属离子偏爱Py 2 ImAm哪个配位点的关键因素是弱酸的存在(或不存在)。更具体地说,弱酸的存在将金属离子引导到Py 2 ImAm的三齿位点,而中性或基本条件会导致配位络合物,其中金属中心被束缚在双齿袋中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号