当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Outcomes With Transcatheter Mitral Valve Repair in the United States
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jacc.2017.09.015 Paul Sorajja , Sreekanth Vemulapalli , Ted Feldman , Michael Mack , David R. Holmes , Amanda Stebbins , Saibal Kar , Vinod Thourani , Gorav Ailawadi
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jacc.2017.09.015 Paul Sorajja , Sreekanth Vemulapalli , Ted Feldman , Michael Mack , David R. Holmes , Amanda Stebbins , Saibal Kar , Vinod Thourani , Gorav Ailawadi
|
BACKGROUND
Post-market surveillance is needed to evaluate the real-world clinical effectiveness and safety of U.S. Food and Drug Administration-approved devices. OBJECTIVES
The authors examined the commercial experience with transcatheter mitral valve repair for the treatment of mitral regurgitation. METHODS
Data from the Society of Thoracic Surgery/American College of Cardiology Transcatheter Valve Therapy Registry on patients commercially treated with transcatheter mitral valve repair were analyzed. The study population consisted of 2,952 patients treated at 145 hospitals between November 2013 and September 2015. In 1,867 patients, data were linked to patient-specific Centers for Medicare and Medicaid Services administrative claims for analyses. RESULTS
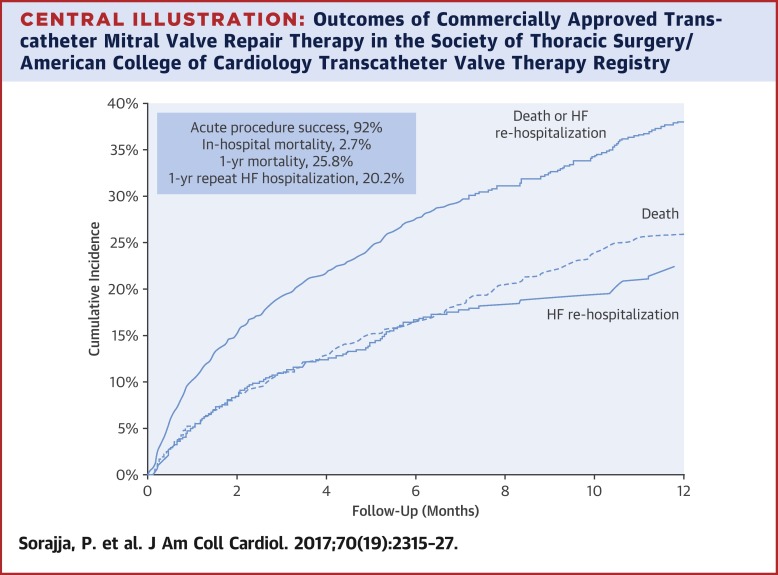
The median age was 82 years (55.8% men), with a median Society of Thoracic Surgery predicted risk of mortality of 6.1% (interquartile range: 3.7% to 9.9%) and 9.2% (interquartile range: 6.0% to 14.1%) for mitral repair and replacement, respectively. Overall, in-hospital mortality was 2.7%. Acute procedure success occurred in 91.8%. Among the patients with Centers for Medicare and Medicaid Services linkage data, the mortality at 30 days and at 1 year was 5.2% and 25.8%, respectively, and repeat hospitalization for heart failure at 1 year occurred in 20.2%. Variables associated with mortality or rehospitalization for heart failure after multivariate adjustment were increasing age, lower baseline left ventricular ejection fraction, worse post-procedural mitral regurgitation, moderate or severe lung disease, dialysis, and severe tricuspid regurgitation. CONCLUSIONS
Our findings demonstrate that commercial transcatheter mitral valve repair is being performed in the United States with acute effectiveness and safety. Our findings may help determine which patients have favorable long-term outcomes with this therapy.
中文翻译:

美国经导管二尖瓣修复的结果
背景 需要进行上市后监测来评估美国食品和药物管理局批准的器械在真实世界中的临床有效性和安全性。目的 作者研究了经导管二尖瓣修复术治疗二尖瓣关闭不全的商业经验。方法 分析了来自胸外科学会/美国心脏病学会经导管瓣膜治疗登记处关于接受经导管二尖瓣修复商业治疗的患者的数据。研究人群包括 2013 年 11 月至 2015 年 9 月期间在 145 家医院接受治疗的 2,952 名患者。在 1,867 名患者中,数据与特定患者的医疗保险和医疗补助服务中心的行政索赔相关联以进行分析。结果 中位年龄为 82 岁(55.8% 男性),胸外科学会预测的二尖瓣修复和置换的死亡风险中位数分别为 6.1%(四分位距:3.7% 至 9.9%)和 9.2%(四分位距:6.0% 至 14.1%)。总体而言,住院死亡率为 2.7%。急性手术成功率为 91.8%。在有医疗保险和医疗补助服务中心联动数据的患者中,30 天和 1 年的死亡率分别为 5.2% 和 25.8%,1 年因心力衰竭再次住院的发生率为 20.2%。多变量调整后与死亡率或心力衰竭再住院相关的变量是年龄增加、基线左心室射血分数较低、术后二尖瓣关闭不全更严重、中度或重度肺病、透析和严重三尖瓣关闭不全。结论我们的研究结果表明,商业经导管二尖瓣修复正在美国进行,具有极高的有效性和安全性。我们的发现可能有助于确定哪些患者使用这种疗法具有良好的长期结果。
更新日期:2017-11-01
中文翻译:

美国经导管二尖瓣修复的结果
背景 需要进行上市后监测来评估美国食品和药物管理局批准的器械在真实世界中的临床有效性和安全性。目的 作者研究了经导管二尖瓣修复术治疗二尖瓣关闭不全的商业经验。方法 分析了来自胸外科学会/美国心脏病学会经导管瓣膜治疗登记处关于接受经导管二尖瓣修复商业治疗的患者的数据。研究人群包括 2013 年 11 月至 2015 年 9 月期间在 145 家医院接受治疗的 2,952 名患者。在 1,867 名患者中,数据与特定患者的医疗保险和医疗补助服务中心的行政索赔相关联以进行分析。结果 中位年龄为 82 岁(55.8% 男性),胸外科学会预测的二尖瓣修复和置换的死亡风险中位数分别为 6.1%(四分位距:3.7% 至 9.9%)和 9.2%(四分位距:6.0% 至 14.1%)。总体而言,住院死亡率为 2.7%。急性手术成功率为 91.8%。在有医疗保险和医疗补助服务中心联动数据的患者中,30 天和 1 年的死亡率分别为 5.2% 和 25.8%,1 年因心力衰竭再次住院的发生率为 20.2%。多变量调整后与死亡率或心力衰竭再住院相关的变量是年龄增加、基线左心室射血分数较低、术后二尖瓣关闭不全更严重、中度或重度肺病、透析和严重三尖瓣关闭不全。结论我们的研究结果表明,商业经导管二尖瓣修复正在美国进行,具有极高的有效性和安全性。我们的发现可能有助于确定哪些患者使用这种疗法具有良好的长期结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号