当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activated Microglia Targeting Dendrimer–Minocycline Conjugate as Therapeutics for Neuroinflammation
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00569 Rishi Sharma 1 , Soo-Young Kim 1 , Anjali Sharma 1 , Zhi Zhang 2 , Siva Pramodh Kambhampati 1 , Sujatha Kannan 1, 2, 3, 4 , Rangaramanujam M. Kannan 1, 3, 4, 5
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00569 Rishi Sharma 1 , Soo-Young Kim 1 , Anjali Sharma 1 , Zhi Zhang 2 , Siva Pramodh Kambhampati 1 , Sujatha Kannan 1, 2, 3, 4 , Rangaramanujam M. Kannan 1, 3, 4, 5
Affiliation
|
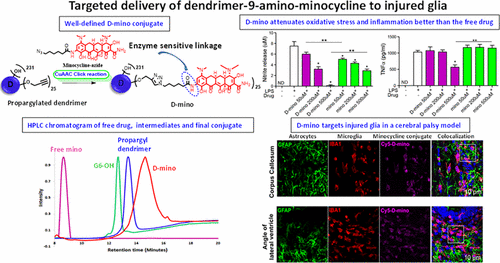
Brain-related disorders have outmatched cancer and cardiovascular diseases worldwide as the leading cause of morbidity and mortality. The lack of effective therapies and the relatively dry central nervous system (CNS) drug pipeline pose formidable challenge. Superior, targeted delivery of current clinically approved drugs may offer significant potential. Minocycline has shown promise for the treatment of neurological diseases owing to its ability to penetrate the blood–brain barrier (BBB) and potency. Despite its potential in the clinic and in preclinical models, the high doses needed to affect a positive therapeutic response have led to side effects. Targeted delivery of minocycline to the injured site and injured cells in the brain can be highly beneficial. Systemically administered hydroxyl poly(amidoamine) (PAMAM) generation-6 (G6) dendrimers have a longer blood circulation time and have been shown to cross the impaired BBB. We have successfully prepared and characterized the in vitro efficacy and in vivo targeting ability of hydroxyl-G6 PAMAM dendrimer–9-amino-minocycline conjugate (D-mino). Minocycline is a challenging drug to carry out chemical transformations due to its inherent instability. We used a combination of a highly efficient and mild copper catalyzed azide–alkyne click reaction (CuAAC) along with microwave energy to conjugate 9-amino-minocycline (mino) to the dendrimer surface via enzyme responsive linkages. D-mino was further evaluated for anti-inflammatory and antioxidant activity in lipopolysaccharides-activated murine microglial cells. D-mino conjugates enhanced the intracellular availability of the drug due to their rapid uptake, suppressed inflammatory cytokine tumor necrosis factor α (TNF-α) production, and reduced oxidative stress by suppressing nitric oxide production, all significantly better than the free drug. Fluorescently labeled dendrimer conjugate (Cy5–D-mino) was systematically administered (intravenous, 55 mg/kg) on postnatal day 1 to rabbit kits with a clinically relevant phenotype of cerebral palsy. The in vivo imaging study indicates that Cy5–D-mino crossed the impaired blood–brain barrier and co-localized with activated microglia at the periventricular white matter areas, including the corpus callosum and the angle of the lateral ventricle, with significant implications for positive therapeutic outcomes. The enhanced efficacy of D-mino, when combined with the inherent neuroinflammation-targeting capability of the PAMAM dendrimers, may provide new opportunities for targeted drug delivery to treat neurological disorders.
中文翻译:

靶向小树胶–米诺环素的活化小胶质细胞结合物可作为神经炎症的治疗药物
与脑有关的疾病已成为全球发病率和死亡率的主要原因,已经超过了癌症和心血管疾病。缺乏有效的治疗方法和相对干燥的中枢神经系统(CNS)药物管道构成了巨大的挑战。当前临床上批准的药物的优越,有针对性的递送可能会提供巨大的潜力。米诺环素具有穿透血脑屏障(BBB)的能力和效力,因此已显示出治疗神经系统疾病的希望。尽管它在临床和临床前模型中具有潜力,但影响积极的治疗反应所需的高剂量已导致副作用。将米诺环素靶向递送至受伤部位和大脑中受损细胞可能非常有益。全身施用的羟基聚(酰胺基胺)(PAMAM)第6代(G6)树状聚合物具有更长的血液循环时间,并且已经证明可以穿越受损的BBB。我们已经成功地制备并表征了羟基G6 PAMAM树状聚合物9-氨基-氨基环素缀合物(D-mino)的体外功效和体内靶向能力。米诺环素因其固有的不稳定性而成为进行化学转化的具有挑战性的药物。我们结合使用了高效,温和的铜催化的叠氮化物-炔烃点击反应(CuAAC)以及微波能,通过酶反应性键合将9-氨基-氨基环素(mino)偶联至树枝状聚合物表面。进一步评估了D-mino在脂多糖激活的鼠小神经胶质细胞中的抗炎和抗氧化活性。D-氨基共轭物由于其快速吸收,抑制炎性细胞因子肿瘤坏死因子α(TNF-α)的产生而提高了药物在细胞内的利用率,并通过抑制一氧化氮的产生而降低了氧化应激,所有这些均显着优于游离药物。荧光标记的树状聚合物结合物(Cy5-D-氨基)于出生后第1天系统性给药(静脉注射,55 mg / kg)给具有临床相关性脑瘫表型的兔子试剂盒。体内成像研究表明,Cy5–D-氨基穿过受损的血脑屏障,并与激活的小胶质细胞共定位于脑室周围白质区域,包括call体和侧脑室角度,对阳性呈显着影响治疗结果。D-mino的功效增强,
更新日期:2017-10-28
中文翻译:

靶向小树胶–米诺环素的活化小胶质细胞结合物可作为神经炎症的治疗药物
与脑有关的疾病已成为全球发病率和死亡率的主要原因,已经超过了癌症和心血管疾病。缺乏有效的治疗方法和相对干燥的中枢神经系统(CNS)药物管道构成了巨大的挑战。当前临床上批准的药物的优越,有针对性的递送可能会提供巨大的潜力。米诺环素具有穿透血脑屏障(BBB)的能力和效力,因此已显示出治疗神经系统疾病的希望。尽管它在临床和临床前模型中具有潜力,但影响积极的治疗反应所需的高剂量已导致副作用。将米诺环素靶向递送至受伤部位和大脑中受损细胞可能非常有益。全身施用的羟基聚(酰胺基胺)(PAMAM)第6代(G6)树状聚合物具有更长的血液循环时间,并且已经证明可以穿越受损的BBB。我们已经成功地制备并表征了羟基G6 PAMAM树状聚合物9-氨基-氨基环素缀合物(D-mino)的体外功效和体内靶向能力。米诺环素因其固有的不稳定性而成为进行化学转化的具有挑战性的药物。我们结合使用了高效,温和的铜催化的叠氮化物-炔烃点击反应(CuAAC)以及微波能,通过酶反应性键合将9-氨基-氨基环素(mino)偶联至树枝状聚合物表面。进一步评估了D-mino在脂多糖激活的鼠小神经胶质细胞中的抗炎和抗氧化活性。D-氨基共轭物由于其快速吸收,抑制炎性细胞因子肿瘤坏死因子α(TNF-α)的产生而提高了药物在细胞内的利用率,并通过抑制一氧化氮的产生而降低了氧化应激,所有这些均显着优于游离药物。荧光标记的树状聚合物结合物(Cy5-D-氨基)于出生后第1天系统性给药(静脉注射,55 mg / kg)给具有临床相关性脑瘫表型的兔子试剂盒。体内成像研究表明,Cy5–D-氨基穿过受损的血脑屏障,并与激活的小胶质细胞共定位于脑室周围白质区域,包括call体和侧脑室角度,对阳性呈显着影响治疗结果。D-mino的功效增强,



























 京公网安备 11010802027423号
京公网安备 11010802027423号