当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ambient‐Light‐Promoted Perfluoroalkylative Cyclization of β,γ‐Unsaturated Hydrazones: Synthesis of Perfluoroalkylated Pyrazolines
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-10-26 , DOI: 10.1002/ejoc.201701201 Jing-miao Yu 1 , Chun Cai 1, 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-10-26 , DOI: 10.1002/ejoc.201701201 Jing-miao Yu 1 , Chun Cai 1, 2
Affiliation
|
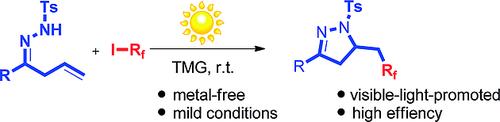
An ambient‐light‐promoted, metal‐free strategy for the direct perfluoroalkylative cyclization of β,γ‐unsaturated hydrazones is reported. This radical process has wide functional‐group tolerance, is performed under mild reaction conditions, and is operationally simple. The perfluoroalkylated pyrazoline products are obtained in good to excellent yields. TMG = 1,1,3,3‐tetramethylguanidine.

中文翻译:

β,γ-不饱和zone的环境光促进全氟烷基化环化:全氟烷基化吡唑啉的合成
报道了一种环境光促进,无金属的策略,可直接对β,γ-不饱和azo进行全氟烷基化环化。该自由基过程具有宽泛的官能团耐受性,可在温和的反应条件下进行,并且操作简单。全氟烷基化的吡唑啉产物以良好至优异的产率获得。TMG = 1,1,3,3-四甲基胍。

更新日期:2017-10-26

中文翻译:

β,γ-不饱和zone的环境光促进全氟烷基化环化:全氟烷基化吡唑啉的合成
报道了一种环境光促进,无金属的策略,可直接对β,γ-不饱和azo进行全氟烷基化环化。该自由基过程具有宽泛的官能团耐受性,可在温和的反应条件下进行,并且操作简单。全氟烷基化的吡唑啉产物以良好至优异的产率获得。TMG = 1,1,3,3-四甲基胍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号