当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potentiality and Synthesis of O- and N-Heterocycles: Pd-Catalyzed Cyclocarbonylative Sonogashira Coupling as a Valuable Route to Phthalans, Isochromans, and Isoindolines
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-10-24 , DOI: 10.1002/ejoc.201701041 Gianluigi Albano 1 , Laura Antonella Aronica 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-10-24 , DOI: 10.1002/ejoc.201701041 Gianluigi Albano 1 , Laura Antonella Aronica 1
Affiliation
|
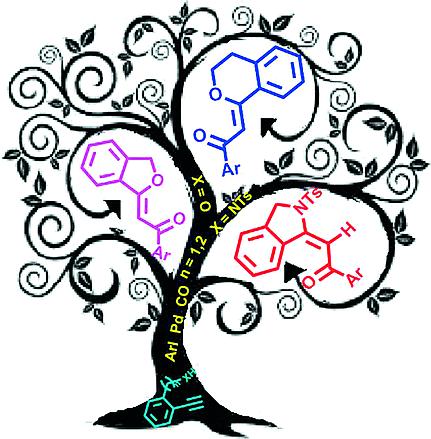
Oxygen and nitrogen heterocycle systems are found in a vast number of natural substrates and biologically active molecules. In particular, phthalan, isochroman, and isoindoline scaffolds are present in many classes of products such as antimycotics, antibiotics, antioxidants, pigments, and fluorophores. Therefore several procedure dedicated to the building of such heterocycles have been developed. In this review a detailed analysis of literature data relating to these scaffolds is described. Particular attention has been devoted to their biological and chemical potentiality, and an in-depth investigation into the most important synthetic methods is reported. Cyclocarbonylative Sonogashira coupling of suitable alcohols and amides has been carefully considered, because it represents a valuable and atom-economic route for the construction of alkylidenephthalans, -isochromans, and -isoindolines.
中文翻译:

O-和 N-杂环的潜力和合成:Pd 催化的环羰基化 Sonogashira 偶联作为通往邻苯二甲酚、异色曼和异二氢吲哚的宝贵途径
氧和氮杂环系统存在于大量天然底物和生物活性分子中。特别是,邻苯二甲酸酯、异色满和异二氢吲哚支架存在于许多类别的产品中,例如抗真菌剂、抗生素、抗氧化剂、色素和荧光团。因此,已经开发了几种专门用于构建此类杂环的程序。在这篇综述中,详细分析了与这些支架相关的文献数据。特别关注它们的生物和化学潜力,并报告了对最重要合成方法的深入研究。已经仔细考虑了合适的醇和酰胺的环羰基化 Sonogashira 偶联,
更新日期:2017-10-24
中文翻译:

O-和 N-杂环的潜力和合成:Pd 催化的环羰基化 Sonogashira 偶联作为通往邻苯二甲酚、异色曼和异二氢吲哚的宝贵途径
氧和氮杂环系统存在于大量天然底物和生物活性分子中。特别是,邻苯二甲酸酯、异色满和异二氢吲哚支架存在于许多类别的产品中,例如抗真菌剂、抗生素、抗氧化剂、色素和荧光团。因此,已经开发了几种专门用于构建此类杂环的程序。在这篇综述中,详细分析了与这些支架相关的文献数据。特别关注它们的生物和化学潜力,并报告了对最重要合成方法的深入研究。已经仔细考虑了合适的醇和酰胺的环羰基化 Sonogashira 偶联,



























 京公网安备 11010802027423号
京公网安备 11010802027423号