Molecular Cell ( IF 16.0 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.molcel.2017.09.037 Satoshi Ishiyama , Atsuya Nishiyama , Yasushi Saeki , Kei Moritsugu , Daichi Morimoto , Luna Yamaguchi , Naoko Arai , Rumie Matsumura , Toru Kawakami , Yuichi Mishima , Hironobu Hojo , Shintaro Shimamura , Fuyuki Ishikawa , Shoji Tajima , Keiji Tanaka , Mariko Ariyoshi , Masahiro Shirakawa , Mitsunori Ikeguchi , Akinori Kidera , Isao Suetake , Kyohei Arita , Makoto Nakanishi
|
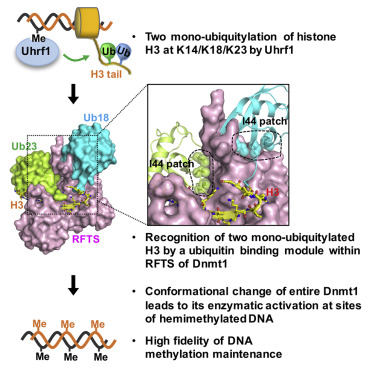
The proper location and timing of Dnmt1 activation are essential for DNA methylation maintenance. We demonstrate here that Dnmt1 utilizes two-mono-ubiquitylated histone H3 as a unique ubiquitin mark for its recruitment to and activation at DNA methylation sites. The crystal structure of the replication foci targeting sequence (RFTS) of Dnmt1 in complex with H3-K18Ub/23Ub reveals striking differences to the known ubiquitin-recognition structures. The two ubiquitins are simultaneously bound to the RFTS with a combination of canonical hydrophobic and atypical hydrophilic interactions. The C-lobe of RFTS, together with the K23Ub surface, also recognizes the N-terminal tail of H3. The binding of H3-K18Ub/23Ub results in spatial rearrangement of two lobes in the RFTS, suggesting the opening of its active site. Actually, incubation of Dnmt1 with H3-K18Ub/23Ub increases its catalytic activity in vitro. Our results therefore shed light on the essential role of a unique ubiquitin-binding module in DNA methylation maintenance.
中文翻译:

Dnmt1阅读器模块的结构与组蛋白H3上独特的两个单泛素标记相结合,揭示了DNA甲基化维持的基础
Dnmt1激活的正确位置和时机对于DNA甲基化的维持至关重要。我们在这里证明Dnmt1利用两个单泛素化的组蛋白H3作为其招募和激活DNA甲基化位点的唯一泛素标记。Dnmt1与H3-K18Ub / 23Ub复合的复制灶靶向序列(RFTS)的晶体结构揭示了与已知的泛素识别结构的显着差异。两种泛素同时通过规范的疏水性和非典型的亲水性相互作用与RFTS结合。RFTS的C瓣与K23Ub表面一起也识别H3的N末端尾巴。H3-K18Ub / 23Ub的结合导致RFTS中两个叶的空间重排,表明其活性位点开放。实际上,体外。因此,我们的结果揭示了独特的泛素结合模块在DNA甲基化维持中的重要作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号