当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deposition of PdPtAu Nanoparticles on Hollow Nanospheres of Fe3 O4 as a New Catalyst for Methanol Electrooxidation: Application in Direct Methanol Fuel Cell
Electroanalysis ( IF 3 ) Pub Date : 2017-10-19 , DOI: 10.1002/elan.201700124 Sara Haghnegahdar 1 , Meissam Noroozifar 1
Electroanalysis ( IF 3 ) Pub Date : 2017-10-19 , DOI: 10.1002/elan.201700124 Sara Haghnegahdar 1 , Meissam Noroozifar 1
Affiliation
|
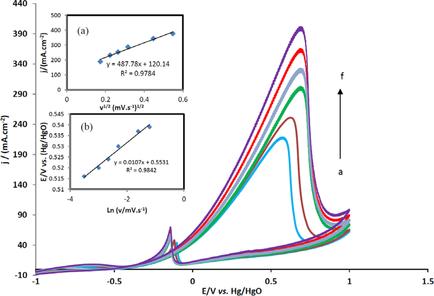
In this study, immobilized hollow nanospheres of Fe3O4 with Palladium, Platinum and Gold nanoparticles (Fe3O4HNS-PdPtAuNPs) was synthesized by hydrothermal and chemical reduction methods and characterized by various techniques such as field emission scanning electron microscopy, energy dispersive analysis of X-rays and elemental mapping images. The electrocatalytic activity of the modified glassy carbon electrode (GCE) with Fe3O4HNS-PdPtAuNPs (GCE/Fe3O4HNS-PdPtAuNPs) toward methanol electrooxidation was investigated by cyclic voltammetry and chronoamperometry in 1 M NaOH solution. According to the results, Fe3O4HNS-PdPtAuNPs catalyst demonstrated the highest efficiency for methanol electrooxidation in comparison with Fe3O4HNS-PdNPs, Fe3O4HNS-PtNPs, Fe3O4HNS-PdAuNPs, Fe3O4HNS-PtAuNPs and Fe3O4HNS-PdPtNPs. The value of electron transfer coefficient (α) and the ratio of current densities (If/Ib) for methanol oxidation on the Fe3O4HNS-PdPtAuNPs/GC catalyst were calculated 0.61 and 5.13, respectively. The reaction order was discovered to be 0.98 for CH3OH. A direct methanol fuel cell was developed with the suggested catalyst under several conditions.
中文翻译:

在 Fe3O4 空心纳米球上沉积 PdPtAu 纳米颗粒作为甲醇电氧化的新催化剂:在直接甲醇燃料电池中的应用
在这项研究中,通过水热和化学还原方法合成了 Fe3O4 与钯、铂和金纳米粒子 (Fe3O4HNS-PdPtAuNPs) 的固定空心纳米球,并通过各种技术进行表征,如场发射扫描电子显微镜、X 射线能量色散分析和元素映射图像。通过循环伏安法和计时电流法在 1 M NaOH 溶液中研究了具有 Fe3O4HNS-PdPtAuNPs(GCE/Fe3O4HNS-PdPtAuNPs)的改性玻璃碳电极(GCE)对甲醇电氧化的电催化活性。结果表明,与 Fe3O4HNS-PdNPs、Fe3O4HNS-PtNPs、Fe3O4HNS-PdAuNPs、Fe3O4HNS-PtAuNPs 和 Fe3O4HNS-PdPtNPs 相比,Fe3O4HNS-PdPtAuNPs 催化剂表现出最高的甲醇电氧化效率。Fe3O4HNS-PdPtAuNPs/GC 催化剂上甲醇氧化的电子转移系数 (α) 值和电流密度比 (If/Ib) 分别计算为 0.61 和 5.13。发现CH 3 OH的反应阶数为0.98。在几种条件下,使用建议的催化剂开发了直接甲醇燃料电池。
更新日期:2017-10-19
中文翻译:

在 Fe3O4 空心纳米球上沉积 PdPtAu 纳米颗粒作为甲醇电氧化的新催化剂:在直接甲醇燃料电池中的应用
在这项研究中,通过水热和化学还原方法合成了 Fe3O4 与钯、铂和金纳米粒子 (Fe3O4HNS-PdPtAuNPs) 的固定空心纳米球,并通过各种技术进行表征,如场发射扫描电子显微镜、X 射线能量色散分析和元素映射图像。通过循环伏安法和计时电流法在 1 M NaOH 溶液中研究了具有 Fe3O4HNS-PdPtAuNPs(GCE/Fe3O4HNS-PdPtAuNPs)的改性玻璃碳电极(GCE)对甲醇电氧化的电催化活性。结果表明,与 Fe3O4HNS-PdNPs、Fe3O4HNS-PtNPs、Fe3O4HNS-PdAuNPs、Fe3O4HNS-PtAuNPs 和 Fe3O4HNS-PdPtNPs 相比,Fe3O4HNS-PdPtAuNPs 催化剂表现出最高的甲醇电氧化效率。Fe3O4HNS-PdPtAuNPs/GC 催化剂上甲醇氧化的电子转移系数 (α) 值和电流密度比 (If/Ib) 分别计算为 0.61 和 5.13。发现CH 3 OH的反应阶数为0.98。在几种条件下,使用建议的催化剂开发了直接甲醇燃料电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号