当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
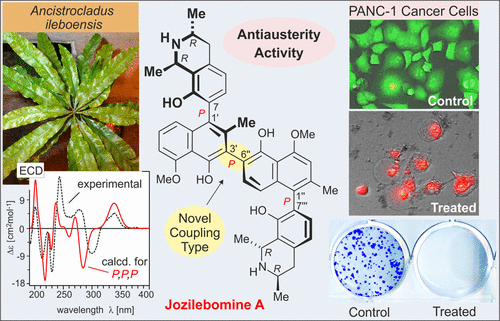
Jozilebomines A and B, Naphthylisoquinoline Dimers from the Congolese Liana Ancistrocladus ileboensis, with Antiausterity Activities against the PANC-1 Human Pancreatic Cancer Cell Line
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00650 Jun Li 1, 2 , Raina Seupel 1 , Torsten Bruhn 1, 3 , Doris Feineis 1 , Marcel Kaiser 4, 5 , Reto Brun 4, 5 , Virima Mudogo 6 , Suresh Awale 7 , Gerhard Bringmann 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00650 Jun Li 1, 2 , Raina Seupel 1 , Torsten Bruhn 1, 3 , Doris Feineis 1 , Marcel Kaiser 4, 5 , Reto Brun 4, 5 , Virima Mudogo 6 , Suresh Awale 7 , Gerhard Bringmann 1
Affiliation
|
Two new naphthylisoquinoline dimers, jozilebomines A (1a) and B (1b), were isolated from the roots of the Congolese plant Ancistrocladus ileboensis, along with the known dimer jozimine A2 (2). These compounds are Dioncophyllaceae-type metabolites, i.e., lacking oxygen functions at C-6 and with an R-configuration at C-3 in their tetrahydroisoquinoline moieties. The dimers 1a and 1b consist of two 7,1′-coupled naphthylisoquinoline monomers linked through an unprecedented 3′,6″-coupling in the binaphthalene core and not, as in 2, via the C-3-positions of the two naphthalene units. Thus, different from the C2-symmetric jozimine A2 (2), the new jozilebomines are constitutionally unsymmetric. The central biaryl axis of each of the three dimers is rotationally hindered, so that 1a, 1b, and 2 possess three consecutive chiral axes. The two jozilebomines have identical constitutions and the same absolute configurations at all four stereogenic centers, but differ from each other in their axial chirality. Their structural elucidation was achieved by HRESIMS, 1D and 2D NMR, oxidative degradation, and experimental and calculated ECD data. They exhibited distinct and specific antiplasmodial activities. All dimers showed potent cytotoxicity against HeLa human cervical cancer cells and preferential cytotoxicity against PANC-1 human pancreatic cancer cells under nutrition-deprived conditions. Furthermore, these dimers significantly inhibited the colony formation of PANC-1 cells, even when exposed to noncytotoxic concentration for a short time. Jozilebomines A (1a) and B (1b) and jozimine A2 (2) represent novel potential candidates for future drug development against pancreatic cancer.
中文翻译:

Jozilebomines A和B,来自刚果藤本植物Ancistrocladus ileboensis的萘基异喹啉二聚体,对PANC-1人胰腺癌细胞系具有抗紧缩活性
从刚果植物Ancistrocladus ileboensis的根中分离出两个新的萘基异喹啉二聚体,jozilebomines A(1a)和B(1b),以及已知的二聚体jozimine A 2(2)。这些化合物是Dioncophyllaceae-type代谢产物,即在它们的四氢异喹啉部分中在C-6处缺乏氧功能并且在C-3处具有R-构型。二聚体1a和1b由两个7,1'偶联的萘基异喹啉单体组成,它们通过在双萘核中空前的3',6''偶联而连接,而不像图2中那样通过两个萘单元的C-3-位置连接。因此,不同于C 2-对称的偶氮苯胺A 2(2),新的偶氮苯胺在结构上是不对称的。三个二聚体中每一个的中心联芳基轴均受旋转阻碍,因此1a,1b和2拥有三个连续的手性轴。两种Jozilebomine在所有四个立体异构中心具有相同的组成和相同的绝对构型,但在轴向手性上彼此不同。通过HRESIMS,1D和2D NMR,氧化降解以及实验和计算得出的ECD数据可以实现对它们的结构阐明。它们表现出独特和特定的抗疟原虫活性。在缺乏营养的条件下,所有二聚体均显示出对HeLa人宫颈癌细胞的强力细胞毒性和对PANC-1人胰腺癌细胞的优先细胞毒性。此外,即使在短时间内暴露于非细胞毒性浓度下,这些二聚体也显着抑制了PANC-1细胞的集落形成。Jozilebomines A(1a)和B(1b)和jozimine A 2(2)代表了针对胰腺癌的未来药物开发的新型潜在候选药物。
更新日期:2017-10-18
中文翻译:

Jozilebomines A和B,来自刚果藤本植物Ancistrocladus ileboensis的萘基异喹啉二聚体,对PANC-1人胰腺癌细胞系具有抗紧缩活性
从刚果植物Ancistrocladus ileboensis的根中分离出两个新的萘基异喹啉二聚体,jozilebomines A(1a)和B(1b),以及已知的二聚体jozimine A 2(2)。这些化合物是Dioncophyllaceae-type代谢产物,即在它们的四氢异喹啉部分中在C-6处缺乏氧功能并且在C-3处具有R-构型。二聚体1a和1b由两个7,1'偶联的萘基异喹啉单体组成,它们通过在双萘核中空前的3',6''偶联而连接,而不像图2中那样通过两个萘单元的C-3-位置连接。因此,不同于C 2-对称的偶氮苯胺A 2(2),新的偶氮苯胺在结构上是不对称的。三个二聚体中每一个的中心联芳基轴均受旋转阻碍,因此1a,1b和2拥有三个连续的手性轴。两种Jozilebomine在所有四个立体异构中心具有相同的组成和相同的绝对构型,但在轴向手性上彼此不同。通过HRESIMS,1D和2D NMR,氧化降解以及实验和计算得出的ECD数据可以实现对它们的结构阐明。它们表现出独特和特定的抗疟原虫活性。在缺乏营养的条件下,所有二聚体均显示出对HeLa人宫颈癌细胞的强力细胞毒性和对PANC-1人胰腺癌细胞的优先细胞毒性。此外,即使在短时间内暴露于非细胞毒性浓度下,这些二聚体也显着抑制了PANC-1细胞的集落形成。Jozilebomines A(1a)和B(1b)和jozimine A 2(2)代表了针对胰腺癌的未来药物开发的新型潜在候选药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号