当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereocontrolled Semisyntheses of Elliptone and 12aβ-Hydroxyelliptone
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00527 David A. Russell 1 , Winston J. S. Fong 1 , David G. Twigg 1 , Hannah F. Sore 1 , David R. Spring 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00527 David A. Russell 1 , Winston J. S. Fong 1 , David G. Twigg 1 , Hannah F. Sore 1 , David R. Spring 1
Affiliation
|
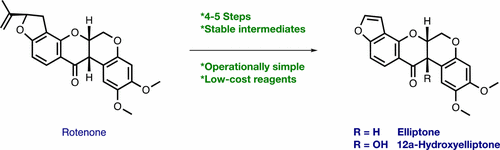
Operationally simple, stereocontrolled semisyntheses of the anticancer rotenoids elliptone and 12aβ-hydroxyelliptone, isolated from Derris elliptica and Derris trifoliata, respectively, are described. Inspired by the work of Singhal, elliptone was prepared from rotenone via a dihydroxylation-oxidative cleavage, chemoselective Baeyer–Villiger oxidation, and acid-catalyzed elimination sequence. Elaboration of elliptone to 12aβ-hydroxyelliptone was achieved via a diastereoselective chromium-mediated Étard-like hydroxylation. The semisynthesis of elliptone constitutes an improvement over previous methods in terms of safety, scalability, and yield, while the first synthesis of 12aβ-hydroxyelliptone is also described.
中文翻译:

立体控制的椭圆和12aβ-羟基磷脂半合成
操作简单,立体控制抗癌鱼藤酮类鱼藤和12aβ-hydroxyelliptone,从分离的semisyntheses鱼藤elliptica和鱼藤,分别进行说明。受辛格(Singhal)的启发,通过二羟化-氧化裂解,化学选择性的拜耳-维利格(Baeyer-Villiger)氧化和酸催化的消除顺序,从鱼藤酮制得了椭圆酮。通过非对映选择性铬介导的类似埃塔德的羟基化作用,将椭圆烷修饰为12aβ-羟基椭圆烷。椭圆的半合成在安全性,可扩展性和产率方面构成了对先前方法的改进,同时还描述了12aβ-羟基椭圆的首次合成。
更新日期:2017-10-17
中文翻译:

立体控制的椭圆和12aβ-羟基磷脂半合成
操作简单,立体控制抗癌鱼藤酮类鱼藤和12aβ-hydroxyelliptone,从分离的semisyntheses鱼藤elliptica和鱼藤,分别进行说明。受辛格(Singhal)的启发,通过二羟化-氧化裂解,化学选择性的拜耳-维利格(Baeyer-Villiger)氧化和酸催化的消除顺序,从鱼藤酮制得了椭圆酮。通过非对映选择性铬介导的类似埃塔德的羟基化作用,将椭圆烷修饰为12aβ-羟基椭圆烷。椭圆的半合成在安全性,可扩展性和产率方面构成了对先前方法的改进,同时还描述了12aβ-羟基椭圆的首次合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号