Immunity ( IF 32.4 ) Pub Date : 2017-10-17 , DOI: 10.1016/j.immuni.2017.09.012 Nicole Glodde , Tobias Bald , Debby van den Boorn-Konijnenberg , Kyohei Nakamura , Jake S. O’Donnell , Sabrina Szczepanski , Maria Brandes , Sarah Eickhoff , Indrajit Das , Naveen Shridhar , Daniel Hinze , Meri Rogava , Tetje C. van der Sluis , Janne J. Ruotsalainen , Evelyn Gaffal , Jennifer Landsberg , Kerstin U. Ludwig , Christoph Wilhelm , Monika Riek-Burchardt , Andreas J. Müller , Christoffer Gebhardt , Richard A. Scolyer , Georgina V. Long , Viktor Janzen , Michele W.L. Teng , Wolfgang Kastenmüller , Massimiliano Mazzone , Mark J. Smyth , Thomas Tüting , Michael Hölzel
|
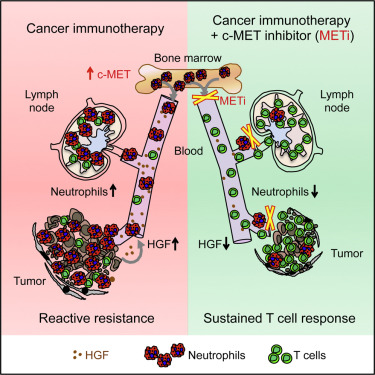
Inhibitors of the receptor tyrosine kinase c-MET are currently used in the clinic to target oncogenic signaling in tumor cells. We found that concomitant c-MET inhibition promoted adoptive T cell transfer and checkpoint immunotherapies in murine cancer models by increasing effector T cell infiltration in tumors. This therapeutic effect was independent of tumor cell-intrinsic c-MET dependence. Mechanistically, c-MET inhibition impaired the reactive mobilization and recruitment of neutrophils into tumors and draining lymph nodes in response to cytotoxic immunotherapies. In the absence of c-MET inhibition, neutrophils recruited to T cell-inflamed microenvironments rapidly acquired immunosuppressive properties, restraining T cell expansion and effector functions. In cancer patients, high serum levels of the c-MET ligand HGF correlated with increasing neutrophil counts and poor responses to checkpoint blockade therapies. Our findings reveal a role for the HGF/c-MET pathway in neutrophil recruitment and function and suggest that c-MET inhibitor co-treatment may improve responses to cancer immunotherapy in settings beyond c-MET-dependent tumors.
中文翻译:

依赖于受体酪氨酸激酶c-MET的反应性中性粒细胞反应限制了癌症的免疫治疗
受体酪氨酸激酶c-MET的抑制剂目前在临床中用于靶向肿瘤细胞中的致癌信号。我们发现伴随c-MET抑制通过增加肿瘤中的效应T细胞浸润而促进了鼠癌模型中的过继T细胞转移和检查点免疫疗法。这种治疗效果独立于肿瘤细胞固有的c-MET依赖性。从机制上讲,c-MET抑制会损害中性粒细胞的反应性动员和募集,使其响应细胞毒性免疫疗法而进入肿瘤并引流淋巴结。在不存在c-MET抑制的情况下,募集到T细胞发炎的微环境中的中性粒细胞迅速获得免疫抑制特性,从而抑制T细胞扩增和效应子功能。在癌症患者中 血清中c-MET配体HGF的高水平与中性粒细胞计数的增加和对检查站封锁疗法的不良反应有关。我们的发现揭示了HGF / c-MET通路在嗜中性白细胞募集和功能中的作用,并暗示c-MET抑制剂的共同治疗可能会改善c-MET依赖性肿瘤以外环境中对癌症免疫疗法的反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号