当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insight into Acyl-ACP Thioesterase toward Substrate Specificity Design
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1021/acschembio.7b00641 Yanbin Feng 1 , Yayue Wang 1, 2 , Jiao Liu 1, 2 , Yinghui Liu 1 , Xupeng Cao 1 , Song Xue 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1021/acschembio.7b00641 Yanbin Feng 1 , Yayue Wang 1, 2 , Jiao Liu 1, 2 , Yinghui Liu 1 , Xupeng Cao 1 , Song Xue 1
Affiliation
|
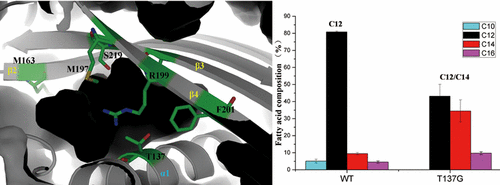
Acyl-ACP thioesterase (TE) catalyzes the hydrolysis of thioester bonds during type II fatty acid synthesis and directly determines fatty acid chain length. Most TEs are responsible for recognition of 16:0 and 18:1 substrates, while specific TEs interrupt acyl-ACP elongation at C8–C14. However, the acyl selection mechanism of TE has not been thoroughly elucidated to date. In this study, the crystal structure of the C12-specific thioesterase FatB from Umbellularia californica, which consists of two independent hotdog domains, was determined. An uncanonical Asp-His-Glu catalytic network was identified on the C-terminal hotdog domain, whereas the substrate binding pocket was determined to be on the N-terminal hotdog domain. Moreover, we elucidated UcFatB’s substrate selection mechanism, which is accommodated by several unconservative amino acids on the β5, β2, and β4 sheets and enclosed by T137 on the α1 helix. On this basis, the C12-specific TE was rationally redesigned toward C14 selectivity by tuning the substrate binding pocket capacity. The T137G mutant demonstrated comparative relative activity on C14 substrates compared to C12 substrates in vitro. Furthermore, the reconstructed UcFatB_T137G achieved C14 fatty acid content up to 40% in contrast to 10% C14 from the wild type in engineered E. coli cells. The unraveled substrate selection mechanism of TE provides a new strategy for tailoring fatty acid synthesis.
中文翻译:

酰基-ACP硫酯酶对底物特异性设计的结构洞察
酰基-ACP硫酯酶(TE)在II型脂肪酸合成过程中催化硫酯键的水解,并直接确定脂肪酸链的长度。大多数TE负责识别16:0和18:1的底物,而特定的TE则中断C8–C14处的酰基ACP延伸。但是,到目前为止,TE的酰基选择机制尚未完全阐明。在这项研究中,来自加利福尼亚伞形藻的C12特异性硫酯酶FatB的晶体结构确定了由两个独立的热狗域组成的。在C末端的热狗结构域上发现了一个非典型的Asp-His-Glu催化网络,而底物结合袋被确定为在N末端的热狗结构域上。此外,我们阐明了UcFatB的底物选择机制,该机制被β5,β2和β4片上的几个非保守氨基酸所容纳,并被α1螺旋上的T137包围。在此基础上,通过调节底物的结合口袋容量,将C12特异性TE合理地重新设计为C14选择性。与体外C12底物相比,T137G突变体在C14底物上具有相对的相对活性。此外,与改造的大肠杆菌细胞中野生型C14的10%相比,重建的UcFatB_T137G的C14脂肪酸含量高达40%。TE的未分类底物选择机制为定制脂肪酸合成提供了新策略。
更新日期:2017-10-16
中文翻译:

酰基-ACP硫酯酶对底物特异性设计的结构洞察
酰基-ACP硫酯酶(TE)在II型脂肪酸合成过程中催化硫酯键的水解,并直接确定脂肪酸链的长度。大多数TE负责识别16:0和18:1的底物,而特定的TE则中断C8–C14处的酰基ACP延伸。但是,到目前为止,TE的酰基选择机制尚未完全阐明。在这项研究中,来自加利福尼亚伞形藻的C12特异性硫酯酶FatB的晶体结构确定了由两个独立的热狗域组成的。在C末端的热狗结构域上发现了一个非典型的Asp-His-Glu催化网络,而底物结合袋被确定为在N末端的热狗结构域上。此外,我们阐明了UcFatB的底物选择机制,该机制被β5,β2和β4片上的几个非保守氨基酸所容纳,并被α1螺旋上的T137包围。在此基础上,通过调节底物的结合口袋容量,将C12特异性TE合理地重新设计为C14选择性。与体外C12底物相比,T137G突变体在C14底物上具有相对的相对活性。此外,与改造的大肠杆菌细胞中野生型C14的10%相比,重建的UcFatB_T137G的C14脂肪酸含量高达40%。TE的未分类底物选择机制为定制脂肪酸合成提供了新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号