当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Toward Personalized Peptide-Based Cancer Nanovaccines: A Facile and Versatile Synthetic Approach
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00502 Hamilton Kakwere 1 , Elizabeth S. Ingham 1 , Riley Allen 1 , Lisa M. Mahakian 1 , Sarah M. Tam 1 , Hua Zhang 1 , Matthew T. Silvestrini 1 , Jamal S. Lewis 1 , Katherine W. Ferrara 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00502 Hamilton Kakwere 1 , Elizabeth S. Ingham 1 , Riley Allen 1 , Lisa M. Mahakian 1 , Sarah M. Tam 1 , Hua Zhang 1 , Matthew T. Silvestrini 1 , Jamal S. Lewis 1 , Katherine W. Ferrara 1
Affiliation
|
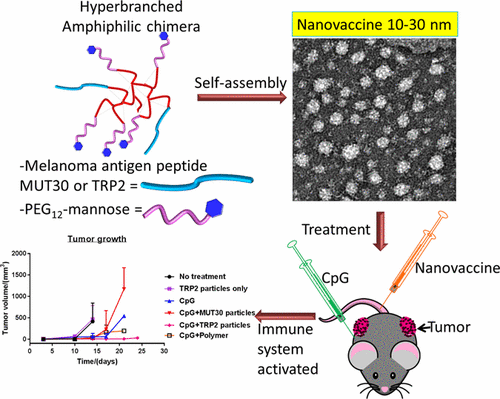
Personalized cancer vaccines (PCVs) are receiving attention as an avenue for cancer immunotherapy. PCVs employ immunogenic peptide epitopes capable of stimulating the immune system to destroy cancer cells with great specificity. Challenges associated with effective delivery of these peptides include poor solubility of hydrophobic sequences, rapid clearance, and poor immunogenicity, among others. The incorporation of peptides into nanoparticles has the potential to overcome these challenges, but the broad range of functionalities found in amino acids presents a challenge to conjugation due to possible interferences and lack of reaction specificity. Herein, a facile and versatile approach to generating nanosized PCVs under mild nonstringent conditions is reported. Following a simple two-step semibatch synthetic approach, amphiphilic hyperbranched polymer–peptide conjugates were prepared by the conjugation of melanoma antigen peptides, either TRP2 (hydrophobic) or MUT30 (hydrophilic), to an alkyne functionalized core via strain-promoted azide–alkyne click chemistry. Self-assembly of the amphiphiles gave spherical nanovaccines (by transmission electron microscopy) with sizes in the range of 10–30 nm (by dynamic light scattering). Fluorescently labeled nanovaccines were prepared to investigate the cellular uptake by antigen presenting cells (dendritic cells), and uptake was confirmed by flow cytometry and microscopy. The TRP2 nanovaccine was taken up the most followed by MUT30 nanoparticles and, finally, nanoparticles without peptide. The nanovaccines showed good biocompatibility against B16–F10 cells, yet the TRP2 peptide showed signs of toxicity, possibly due to its hydrophobicity. A test for immunogenicity revealed that the nanovaccines were poorly immunogenic, implying the need for an adjuvant when administered in vivo. Treatment of mice with melanoma tumors showed that in combination with adjuvant, CpG, groups with the peptide nanovaccines slowed tumor growth and improved survival (up to 24 days, TRP2) compared to the untreated group (14 days).
中文翻译:

走向个性化的基于肽的癌症纳米疫苗:一种简便而多功能的合成方法
个性化癌症疫苗(PCV)作为癌症免疫疗法的一种途径正受到关注。PCV采用能够刺激免疫系统以高度特异性破坏癌细胞的免疫原性肽表位。与这些肽的有效递送相关的挑战包括疏水序列的溶解性差,快速清除和免疫原性差等。将肽掺入纳米颗粒具有克服这些挑战的潜力,但是由于可能的干扰和缺乏反应特异性,氨基酸中发现的广泛功能性对缀合提出了挑战。本文中,报道了在温和的非严格条件下产生纳米级PCV的简便且通用的方法。按照简单的两步半批量综合方法,通过将黑色素瘤抗原肽TRP2(疏水性的)或MUT30(亲水性的)与黑素瘤抗原肽通过应变促进的叠氮化物-炔烃点击化学偶联到炔烃官能化的核上来制备两亲性超支化聚合物-肽偶联物。两亲物的自组装产生了球形纳米疫苗(通过透射电子显微镜),尺寸在10–30 nm范围内(通过动态光散射)。制备荧光标记的纳米疫苗以研究抗原呈递细胞(树突状细胞)对细胞的摄取,并通过流式细胞仪和显微镜检查确认摄取。摄取最多的是TRP2纳米疫苗,其次是MUT30纳米颗粒,最后是不含肽的纳米颗粒。纳米疫苗对B16–F10细胞显示出良好的生物相容性,但TRP2肽显示出毒性迹象,可能是由于其疏水性。免疫原性测试表明,纳米疫苗的免疫原性较差,这意味着在体内给药时需要佐剂。黑色素瘤肿瘤小鼠的治疗显示,与未治疗组(14天)相比,与肽CpG佐剂联合使用的组,纳米肽疫苗减慢了肿瘤的生长,提高了存活率(长达24天,TRP2)。
更新日期:2017-10-13
中文翻译:

走向个性化的基于肽的癌症纳米疫苗:一种简便而多功能的合成方法
个性化癌症疫苗(PCV)作为癌症免疫疗法的一种途径正受到关注。PCV采用能够刺激免疫系统以高度特异性破坏癌细胞的免疫原性肽表位。与这些肽的有效递送相关的挑战包括疏水序列的溶解性差,快速清除和免疫原性差等。将肽掺入纳米颗粒具有克服这些挑战的潜力,但是由于可能的干扰和缺乏反应特异性,氨基酸中发现的广泛功能性对缀合提出了挑战。本文中,报道了在温和的非严格条件下产生纳米级PCV的简便且通用的方法。按照简单的两步半批量综合方法,通过将黑色素瘤抗原肽TRP2(疏水性的)或MUT30(亲水性的)与黑素瘤抗原肽通过应变促进的叠氮化物-炔烃点击化学偶联到炔烃官能化的核上来制备两亲性超支化聚合物-肽偶联物。两亲物的自组装产生了球形纳米疫苗(通过透射电子显微镜),尺寸在10–30 nm范围内(通过动态光散射)。制备荧光标记的纳米疫苗以研究抗原呈递细胞(树突状细胞)对细胞的摄取,并通过流式细胞仪和显微镜检查确认摄取。摄取最多的是TRP2纳米疫苗,其次是MUT30纳米颗粒,最后是不含肽的纳米颗粒。纳米疫苗对B16–F10细胞显示出良好的生物相容性,但TRP2肽显示出毒性迹象,可能是由于其疏水性。免疫原性测试表明,纳米疫苗的免疫原性较差,这意味着在体内给药时需要佐剂。黑色素瘤肿瘤小鼠的治疗显示,与未治疗组(14天)相比,与肽CpG佐剂联合使用的组,纳米肽疫苗减慢了肿瘤的生长,提高了存活率(长达24天,TRP2)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号