当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Assembled Tumor-Penetrating Peptide-Modified Poly(l-γ-glutamylglutamine)–Paclitaxel Nanoparticles Based on Hydrophobic Interaction for the Treatment of Glioblastoma
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00519 Jing Yu 1 , Lei Sun 1 , Jinge Zhou 1 , Lipeng Gao 1 , Lijuan Nan 1 , Shimin Zhao 1 , Ting Peng 1 , Lin Han 1 , Jing Wang 1 , Weiyue Lu 2 , Lin Zhang 3 , Yiting Wang 1 , Zhiqiang Yan 1 , Lei Yu 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00519 Jing Yu 1 , Lei Sun 1 , Jinge Zhou 1 , Lipeng Gao 1 , Lijuan Nan 1 , Shimin Zhao 1 , Ting Peng 1 , Lin Han 1 , Jing Wang 1 , Weiyue Lu 2 , Lin Zhang 3 , Yiting Wang 1 , Zhiqiang Yan 1 , Lei Yu 1
Affiliation
|
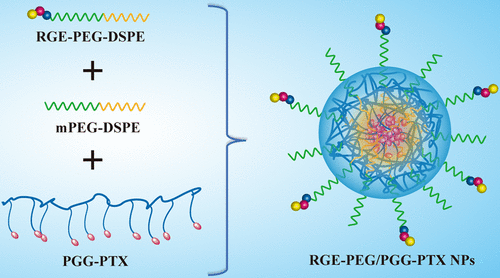
To enhance the tumor-penetrating ability and targeting therapeutic effect of polymer–drug conjugates (PDCs), tumor-penetrating peptide RGERPPR (RGE) modified and PEGylated poly(l-γ-glutamylglutamine)–paclitaxel (PGG–PTX) nanoparticles (RGE–PEG/PGG–PTX NPs) were prepared by using a so-called “modular” design strategy. In brief, a RGERPPR-conjugated targeting material, DSPE–PEG–RGERPPR, was first synthesized and assembled with PGG–PTX into RGE–PEG/PGG–PTX NPs based on the hydrophobic interaction between the groups of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) and PTX. The NPs exhibited a uniform spherical morphology with particle size of around 90 nm, as shown by the dynamic light scattering and transmission electron microscopy results. The NPs showed good in vitro stability at 4 °C for over 3 weeks, sustained drug release within 120 h, and good hemocompatibility. The cellular-uptake study displayed that the NPs showed increased uptake by U87 MG cells and human umbilical vein endothelial cells (HUVECs) compared to the unmodified PGG–PTX. The cytotoxicity test demonstrated that RGE–PEG/PGG–PTX NPs produced a stronger growth inhibitory effect against U87 MG cells and HUVECs than PGG–PTX, which was consistent with the cellular uptake results. Finally, the pharmacodynamic study proved that RGE–PEG/PGG–PTX NPs significantly prolonged the median survival time of nude mice bearing intracranial glioblastoma. The results indicated the effectiveness of RGE–PEG/PGG–PTX NPs in the treatment of glioblastoma as well as the feasibility of the “modular” design strategy in the preparation of active-targeting PDCs.
中文翻译:

疏水相互作用的自组装肿瘤穿透肽修饰的聚(l-γ-谷氨酰胺基)-紫杉醇纳米粒子治疗胶质母细胞瘤
为了增强聚合物-药物偶联物(PDC)的肿瘤穿透能力和靶向治疗效果,肿瘤穿透肽RGERPPR(RGE)修饰了聚乙二醇化聚(1 -γ-谷氨酰胺谷氨酰胺)-紫杉醇(PGG-PTX)纳米颗粒(RGE PEG / PGG–PTX NPs是通过使用所谓的“模块化”设计策略制备的。简而言之,首先合成RGERPPR偶联的靶向材料DSPE–PEG–RGERPPR,然后根据1,2-二硬脂酰-sn基团之间的疏水相互作用将PGG–PTX与RGE–PEG / PGG–PTX NPs组装在一起。-甘油-3-磷酸乙醇胺(DSPE)和PTX。如动态光散射和透射电子显微镜结果所示,NPs表现出均匀的球形形态,粒径约为90 nm。NP在4°C的条件下在3周内显示出良好的体外稳定性,在120 h内持续释放药物,并且具有良好的血液相容性。细胞摄取研究表明,与未修饰的PGG-PTX相比,NPs显示U87 MG细胞和人脐静脉内皮细胞(HUVEC)的摄取增加。细胞毒性测试表明,RGE-PEG / PGG-PTX NPs对P87-PTX的U87 MG细胞和HUVECs产生更强的生长抑制作用,这与细胞摄取的结果一致。最后,药效学研究证明,RGE-PEG / PGG-PTX NPs显着延长了颅内成胶质细胞瘤裸鼠的中位生存时间。结果表明,RGE-PEG / PGG-PTX NPs在治疗胶质母细胞瘤中的有效性以及“模块化”设计策略在制备靶向活性PDCs中的可行性。
更新日期:2017-10-10
中文翻译:

疏水相互作用的自组装肿瘤穿透肽修饰的聚(l-γ-谷氨酰胺基)-紫杉醇纳米粒子治疗胶质母细胞瘤
为了增强聚合物-药物偶联物(PDC)的肿瘤穿透能力和靶向治疗效果,肿瘤穿透肽RGERPPR(RGE)修饰了聚乙二醇化聚(1 -γ-谷氨酰胺谷氨酰胺)-紫杉醇(PGG-PTX)纳米颗粒(RGE PEG / PGG–PTX NPs是通过使用所谓的“模块化”设计策略制备的。简而言之,首先合成RGERPPR偶联的靶向材料DSPE–PEG–RGERPPR,然后根据1,2-二硬脂酰-sn基团之间的疏水相互作用将PGG–PTX与RGE–PEG / PGG–PTX NPs组装在一起。-甘油-3-磷酸乙醇胺(DSPE)和PTX。如动态光散射和透射电子显微镜结果所示,NPs表现出均匀的球形形态,粒径约为90 nm。NP在4°C的条件下在3周内显示出良好的体外稳定性,在120 h内持续释放药物,并且具有良好的血液相容性。细胞摄取研究表明,与未修饰的PGG-PTX相比,NPs显示U87 MG细胞和人脐静脉内皮细胞(HUVEC)的摄取增加。细胞毒性测试表明,RGE-PEG / PGG-PTX NPs对P87-PTX的U87 MG细胞和HUVECs产生更强的生长抑制作用,这与细胞摄取的结果一致。最后,药效学研究证明,RGE-PEG / PGG-PTX NPs显着延长了颅内成胶质细胞瘤裸鼠的中位生存时间。结果表明,RGE-PEG / PGG-PTX NPs在治疗胶质母细胞瘤中的有效性以及“模块化”设计策略在制备靶向活性PDCs中的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号