当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oligomerization State of CXCL4 Chemokines Regulates G Protein-Coupled Receptor Activation
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1021/acschembio.7b00704 Ya-Ping Chen , Hsin-Li Wu , Kevin Boyé 1, 2 , Chen-Ya Pan , Yi-Chen Chen , Nadège Pujol 1, 2 , Chun-Wei Lin , Liang-Yuan Chiu , Clotilde Billottet 1, 2 , Isabel D. Alves 2, 3 , Andreas Bikfalvi 1, 2 , Shih-Che Sue
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1021/acschembio.7b00704 Ya-Ping Chen , Hsin-Li Wu , Kevin Boyé 1, 2 , Chen-Ya Pan , Yi-Chen Chen , Nadège Pujol 1, 2 , Chun-Wei Lin , Liang-Yuan Chiu , Clotilde Billottet 1, 2 , Isabel D. Alves 2, 3 , Andreas Bikfalvi 1, 2 , Shih-Che Sue
Affiliation
|
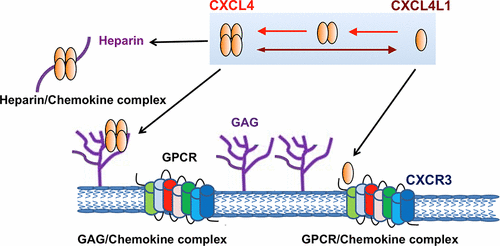
CXCL4 chemokines have antiangiogenic properties, mediated by different mechanisms, including CXCR3 receptor activation. Chemokines have distinct oligomerization states that are correlated with their biological functions. CXCL4 exists as a stable tetramer under physiological conditions. It is unclear whether the oligomerization state impacts CXCL4-receptor interaction. We found that the CXCL4 tetramer is sensitive to pH and salt concentration. Residues Glu28 and Lys50 were important for tetramer formation, and the first β-strand and the C-terminal helix are critical for dimerization. By mutating the critical residues responsible for oligomerization, we generated CXCL4 mutants that behave as dimers or monomers under neutral/physiological conditions. The CXCL4 monomer acts as the minimal active unit for interacting CXCR3A, and sulfation of N-terminal tyrosine residues on the receptor is important for binding. Noticeably, CXCL4L1, a CXCL4 variant that differs by three residues in the C-terminal helix, could activate CXCR3A. CXCL4L1 showed a higher tendency to dissociate into monomers, but native CXCL4 did not. This result indicates that monomeric CXCL4 behaves like CXCL4L1. Thus, in this chemokine family, being in the monomeric state seems critical for interaction with CXCR3A.
中文翻译:

CXCL4趋化因子的低聚状态调节G蛋白偶联的受体激活。
CXCL4趋化因子具有抗血管生成特性,可通过不同的机制介导,包括CXCR3受体激活。趋化因子具有明显的低聚状态,与它们的生物学功能有关。CXCL4在生理条件下以稳定的四聚体形式存在。尚不清楚低聚状态是否影响CXCL4-受体相互作用。我们发现CXCL4四聚体对pH和盐浓度敏感。残基Glu28和Lys50对于四聚体形成很重要,而第一个β链和C末端螺旋对于二聚化至关重要。通过突变负责寡聚的关键残基,我们生成了在中性/生理条件下表现为二聚体或单体形式的CXCL4突变体。CXCL4单体是与CXCR3A相互作用的最小活性单元,受体上N末端酪氨酸残基的硫酸化对于结合很重要。值得注意的是,CXCL4L1是一种CXCL4变体,其C末端螺旋中的三个残基不同,可以激活CXCR3A。CXCL4L1显示更高的离解成单体的趋势,但天然CXCL4没有。此结果表明单体CXCL4的行为类似于CXCL4L1。因此,在这个趋化因子家族中,处于单体状态似乎对于与CXCR3A相互作用至关重要。
更新日期:2017-10-04
中文翻译:

CXCL4趋化因子的低聚状态调节G蛋白偶联的受体激活。
CXCL4趋化因子具有抗血管生成特性,可通过不同的机制介导,包括CXCR3受体激活。趋化因子具有明显的低聚状态,与它们的生物学功能有关。CXCL4在生理条件下以稳定的四聚体形式存在。尚不清楚低聚状态是否影响CXCL4-受体相互作用。我们发现CXCL4四聚体对pH和盐浓度敏感。残基Glu28和Lys50对于四聚体形成很重要,而第一个β链和C末端螺旋对于二聚化至关重要。通过突变负责寡聚的关键残基,我们生成了在中性/生理条件下表现为二聚体或单体形式的CXCL4突变体。CXCL4单体是与CXCR3A相互作用的最小活性单元,受体上N末端酪氨酸残基的硫酸化对于结合很重要。值得注意的是,CXCL4L1是一种CXCL4变体,其C末端螺旋中的三个残基不同,可以激活CXCR3A。CXCL4L1显示更高的离解成单体的趋势,但天然CXCL4没有。此结果表明单体CXCL4的行为类似于CXCL4L1。因此,在这个趋化因子家族中,处于单体状态似乎对于与CXCR3A相互作用至关重要。

























 京公网安备 11010802027423号
京公网安备 11010802027423号