PLOS Medicine ( IF 15.8 ) Pub Date : 2017-09-26 , DOI: 10.1371/journal.pmed.1002390 Caroline A. Crowther , Pat Ashwood , Andrew J. McPhee , Vicki Flenady , Thach Tran , Jodie M. Dodd , Jeffrey S. Robinson ,
|
Background
Neonatal respiratory distress syndrome, as a consequence of preterm birth, is a major cause of early mortality and morbidity. The withdrawal of progesterone, either actual or functional, is thought to be an antecedent to the onset of labour. There remains limited information on clinically relevant health outcomes as to whether vaginal progesterone may be of benefit for pregnant women with a history of a previous preterm birth, who are at high risk of a recurrence. Our primary aim was to assess whether the use of vaginal progesterone pessaries in women with a history of previous spontaneous preterm birth reduced the risk and severity of respiratory distress syndrome in their infants, with secondary aims of examining the effects on other neonatal morbidities and maternal health and assessing the adverse effects of treatment.
Methods
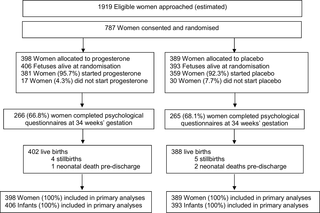
Women with a live singleton or twin pregnancy between 18 to <24 weeks’ gestation and a history of prior preterm birth at less than 37 weeks’ gestation in the preceding pregnancy, where labour occurred spontaneously or in association with cervical incompetence or following preterm prelabour rupture of the membranes, were eligible. Women were recruited from 39 Australian, New Zealand, and Canadian maternity hospitals and assigned by randomisation to vaginal progesterone pessaries (equivalent to 100 mg vaginal progesterone) (n = 398) or placebo (n = 389). Participants and investigators were masked to the treatment allocation. The primary outcome was respiratory distress syndrome and severity. Secondary outcomes were other respiratory morbidities; other adverse neonatal outcomes; adverse outcomes for the woman, especially related to preterm birth; and side effects of progesterone treatment. Data were analysed for all the 787 women (100%) randomised and their 799 infants.
Findings
Most women used their allocated study treatment (740 women, 94.0%), with median use similar for both study groups (51.0 days, interquartile range [IQR] 28.0–69.0, in the progesterone group versus 52.0 days, IQR 27.0–76.0, in the placebo group). The incidence of respiratory distress syndrome was similar in both study groups—10.5% (42/402) in the progesterone group and 10.6% (41/388) in the placebo group (adjusted relative risk [RR] 0.98, 95% confidence interval [CI] 0.64–1.49, p = 0.912)—as was the severity of any neonatal respiratory disease (adjusted treatment effect 1.02, 95% CI 0.69–1.53, p = 0.905). No differences were seen between study groups for other respiratory morbidities and adverse infant outcomes, including serious infant composite outcome (155/406 [38.2%] in the progesterone group and 152/393 [38.7%] in the placebo group, adjusted RR 0.98, 95% CI 0.82–1.17, p = 0.798). The proportion of infants born before 37 weeks’ gestation was similar in both study groups (148/406 [36.5%] in the progesterone group and 146/393 [37.2%] in the placebo group, adjusted RR 0.97, 95% CI 0.81–1.17, p = 0.765). A similar proportion of women in both study groups had maternal morbidities, especially those related to preterm birth, or experienced side effects of treatment. In 9.9% (39/394) of the women in the progesterone group and 7.3% (28/382) of the women in the placebo group, treatment was stopped because of side effects (adjusted RR 1.35, 95% CI 0.85–2.15, p = 0.204). The main limitation of the study was that almost 9% of the women did not start the medication or forgot to use it 3 or more times a week.
Conclusions
Our results do not support the use of vaginal progesterone pessaries in women with a history of a previous spontaneous preterm birth to reduce the risk of neonatal respiratory distress syndrome or other neonatal and maternal morbidities related to preterm birth. Individual participant data meta-analysis of the relevant trials may identify specific women for whom vaginal progesterone might be of benefit.
Trial registration
Current Clinical Trials ISRCTN20269066.
中文翻译:

早产孕妇的阴道孕酮阴道栓可预防新生儿呼吸窘迫综合征(进展研究):一项多中心,随机,安慰剂对照试验
背景
早产导致的新生儿呼吸窘迫综合征是早期死亡和发病的主要原因。孕激素的撤回,无论是实际的还是功能性的,都被认为是引产的先决条件。关于阴道黄体酮是否对具有高复发风险的早产儿史的孕妇是否有益,关于临床相关健康结果的信息仍然有限。我们的主要目的是评估在有自发早产史的妇女中使用阴道孕酮是否能降低其婴儿呼吸窘迫综合征的风险和严重程度,其次要目的是研究其对其他新生儿发病率和孕产妇健康的影响并评估治疗的不良反应。
方法
单胎或双胎活产妊娠在18到<24周之间,并且在先前的妊娠中少于37周的妊娠中有早产史的妇女,其中的劳动是自发发生的或与宫颈功能不全或早产先兆破裂有关。的膜是合格的。从39所澳大利亚,新西兰和加拿大的妇产科医院招募的妇女,随机分配到阴道黄体酮子宫托(相当于100毫克阴道黄体酮)(n = 398)或安慰剂(n= 389)。参加者和研究者被掩盖了治疗分配。主要结果是呼吸窘迫综合征和严重程度。次要结果是其他呼吸道疾病。其他不利的新生儿结局;对妇女的不良后果,特别是与早产有关;和黄体酮治疗的副作用。分析了所有787名(100%)随机分组的妇女及其799名婴儿的数据。
发现
大多数妇女使用分配的研究治疗方法(740名妇女,占94.0%),两个研究组的中位数使用率相似(孕酮组为51.0天,四分位距[IQR] 28.0–69.0,而孕酮组为52.0天,IQR 27.0–76.0)。安慰剂组)。两个研究组的呼吸窘迫综合征的发生率相似,黄体酮组为10.5%(42/402),安慰剂组为10.6%(41/388)(调整后相对危险度[RR] 0.98,置信区间95%[ CI] 0.64–1.49,p = 0.912)—新生儿呼吸系统疾病的严重程度也是如此(调整后的治疗效果1.02,95%CI 0.69–1.53,p= 0.905)。研究组之间在其他呼吸道疾病和不利的婴儿结局方面未见差异,包括严重的婴儿综合结局(孕酮组为155/406 [38.2%],安慰剂组为152/393 [38.7%],校正后的RR 0.98, 95%CI 0.82-1.17,p = 0.798)。两个研究组在妊娠37周之前出生的婴儿比例相似(孕酮组为148/406 [36.5%],安慰剂组为146/393 [37.2%],校正后RR 0.97,95%CI 0.81– 1.17,页= 0.765)。在两个研究组中,有类似比例的妇女患有母亲病,尤其是与早产有关的疾病,或经历过治疗的副作用。在黄体酮组中有9.9%(39/394)的妇女和在安慰剂组中有7.3%(28/382)的妇女因副作用而停止治疗(校正后的相对危险度1.35、95%CI 0.85–2.15,p = 0.204)。该研究的主要局限性在于,几乎9%的女性没有开始服用药物或忘记每周服用3次或更多次。
结论
我们的研究结果不支持在有自发早产史的女性中使用阴道黄体酮子宫托,以减少新生儿呼吸窘迫综合征或其他与早产有关的新生儿和母亲发病的风险。相关试验的个体参与者数据荟萃分析可以确定阴道孕酮可能对其有益的特定女性。
试用注册
当前临床试验ISRCTN20269066。


























 京公网安备 11010802027423号
京公网安备 11010802027423号