当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption Behavior of Asphaltenes and Resins on Kaolinite
Energy & Fuels ( IF 5.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.energyfuels.7b01695 Aristeidis Tsiamis 1 , Spencer E. Taylor 1
Energy & Fuels ( IF 5.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.energyfuels.7b01695 Aristeidis Tsiamis 1 , Spencer E. Taylor 1
Affiliation
|
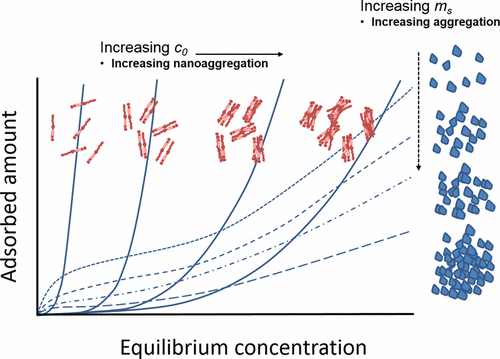
Recent studies have shown that n-C7-precipitated asphaltenes adsorb onto nanoparticles to produce isotherms that are significantly influenced by the dispersed states of both the adsorbate and the adsorbent. In the present work, we investigate this behavior further by determining the adsorption of asphaltene and resin fractions isolated from four different sources onto kaolinite using the depletion method in toluene. Treated conventionally (amount adsorbed, Γ, versus equilibrium bulk concentration, ce), adsorption isotherms for fixed initial concentrations (c0) of C5 and C7 asphaltenes and variable kaolinite mass (ms) are found to be Type I as classified by IUPAC, whereas under the same experimental conditions C5–C7 resins exhibit Type III behavior. By fixing ms and varying c0, however, Type II isotherms are produced by the resins. All of the adsorption results for the same fraction type were found to be very similar, irrespective of the source. The Types I and III isotherms are described very well by the thermodynamic solid–liquid equilibrium (SLE) model of Montoya et al. ( Energy Fuels 2014, 28, 4963−4975) based on the association theory of Talu and Meunier ( AIChE J. 1996, 42, 809−819). Individual isotherms (Γ versus ce) are well-fitted by a shifted Langmuir equation for asphaltenes and by a general Freundlich (power law) relationship for resins. The SLE results verify that in toluene solution the adsorption behavior is complicated by concentration-dependent nanoaggregation of asphaltene species, whereas resin–resin interactions are weaker, but accompanied by adsorbent particle aggregation. On the other hand, when the adsorption data for each fraction type is replotted in terms of the ratio of the experimental parameter c0/ms, as originally done by Wang et al. ( Colloids Surfaces A: Physicochem. Eng. Aspects 2016, 504, 280−286), each set of data merges to a single isotherm which is reasonably well-approximated by a Langmuir-type relationship (we term this a “pseudo-Langmuir equation”), which allows the maximum adsorption to be determined for the different adsorbate/adsorbent systems. The average maximum adsorbed amounts calculated in this way for each of the component types are very similar, being slightly larger for C7A compared with C5A, with the values for the C5–C7R fractions being generally lower and more variable, possibly reflecting some source dependence.
中文翻译:

沥青质和树脂在高岭土上的吸附行为
最近的研究表明,n -C7沉淀的沥青质吸附到纳米颗粒上,产生等温线,该等温线受吸附物和吸附剂的分散状态影响很大。在目前的工作中,我们通过使用甲苯中的耗竭方法,确定从四种不同来源分离出的沥青质和树脂级分在高岭石上的吸附,从而进一步研究这种行为。常规处理(吸附量Γ,相对于平衡体积浓度c e),对于C5和C7沥青质的固定初始浓度(c 0)和高岭石质量可变(m s),吸附等温线)被IUPAC归类为I型,而在相同的实验条件下,C5-C7树脂表现出III型行为。然而,通过固定m s和改变c 0,树脂会产生II型等温线。发现相同馏分类型的所有吸附结果都非常相似,而与来源无关。Montoya等人的热力学固液平衡(SLE)模型很好地描述了I型和III型等温线。(能源燃料 2014年, 28岁, 基于Talu和Meunier(AIChE J. 1996年, 42, 809-819)。单个等温线(Γ与c e)通过沥青质的移位Langmuir方程和树脂的一般Freundlich(幂定律)关系很好地拟合。SLE结果证明,在甲苯溶液中,沥青质物种的浓度依赖性纳米聚集使吸附行为复杂化,而树脂与树脂之间的相互作用较弱,但伴随有吸附剂颗粒聚集。另一方面,当每种馏分类型的吸附数据按照实验参数c 0 / m s的比例重新绘制时,正如Wang等人最初所做的那样。(胶体表面A:物理化学工程方面 2016年, 504, 280-286),每组数据合并为一个等温线,该等温线通过Langmuir型关系合理地近似(我们称其为“伪Langmuir方程”),从而可以确定不同吸附的最大吸附量吸附剂/吸附剂系统。以这种方式计算出的每种组分类型的平均最大吸附量非常相似,与C5A相比,C7A的吸附量略大,C5-C7R馏分的值通常较低且变化较大,可能反映了某些来源的依赖性。
更新日期:2017-09-26
中文翻译:

沥青质和树脂在高岭土上的吸附行为
最近的研究表明,n -C7沉淀的沥青质吸附到纳米颗粒上,产生等温线,该等温线受吸附物和吸附剂的分散状态影响很大。在目前的工作中,我们通过使用甲苯中的耗竭方法,确定从四种不同来源分离出的沥青质和树脂级分在高岭石上的吸附,从而进一步研究这种行为。常规处理(吸附量Γ,相对于平衡体积浓度c e),对于C5和C7沥青质的固定初始浓度(c 0)和高岭石质量可变(m s),吸附等温线)被IUPAC归类为I型,而在相同的实验条件下,C5-C7树脂表现出III型行为。然而,通过固定m s和改变c 0,树脂会产生II型等温线。发现相同馏分类型的所有吸附结果都非常相似,而与来源无关。Montoya等人的热力学固液平衡(SLE)模型很好地描述了I型和III型等温线。(能源燃料



























 京公网安备 11010802027423号
京公网安备 11010802027423号