当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
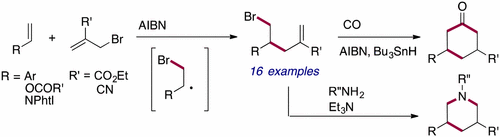
Bromoallylation of Alkenes Leading to 4-Alkenyl Bromides Based on Trapping of β-Bromoalkyl Radicals
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.orglett.7b02471 Takashi Kippo,Kanako Hamaoka,Mitsuhiro Ueda,Takahide Fukuyama,Ilhyong Ryu
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.orglett.7b02471 Takashi Kippo,Kanako Hamaoka,Mitsuhiro Ueda,Takahide Fukuyama,Ilhyong Ryu
|
A radical-chain addition of allyl bromides to aryl alkenes, vinyl ester, and vinyl phthalimide was studied in which elusive β-bromoalkyl radicals were trapped efficiently to give 5-bromo-1-pentenes in good to high yields (16 examples). A subsequent carbonylative radical cyclization with AIBN/Bu3SnH/CO was successful in giving the corresponding 3,5-disubstituted cyclohexanone derivatives in moderate yields. Synthesis of a piperidine ring was also successful by subsequent reaction with primary amine.
中文翻译:

基于捕获的β-溴代烷基自由基的烯烃的溴代芳基化反应,生成4-烯基溴化物
研究了烯丙基溴在芳基烯烃,乙烯基酯和乙烯基邻苯二甲酰亚胺上的自由基链加成反应,其中有效捕集难以捉摸的β-溴烷基自由基,以高到高收率得到5-溴-1-戊烯(16个实例)。随后用AIBN / Bu 3 SnH / CO进行的羰基自由基环化成功地以中等收率得到了相应的3,5-二取代的环己酮衍生物。哌啶环的合成也通过随后与伯胺反应而成功完成。
更新日期:2017-09-25
中文翻译:

基于捕获的β-溴代烷基自由基的烯烃的溴代芳基化反应,生成4-烯基溴化物
研究了烯丙基溴在芳基烯烃,乙烯基酯和乙烯基邻苯二甲酰亚胺上的自由基链加成反应,其中有效捕集难以捉摸的β-溴烷基自由基,以高到高收率得到5-溴-1-戊烯(16个实例)。随后用AIBN / Bu 3 SnH / CO进行的羰基自由基环化成功地以中等收率得到了相应的3,5-二取代的环己酮衍生物。哌啶环的合成也通过随后与伯胺反应而成功完成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号